Label: LOPERAMIDE HYDROCHLORIDE tablet
- NDC Code(s): 63868-338-12, 63868-338-24, 63868-338-60
- Packager: Chain Drug Marketing Association Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
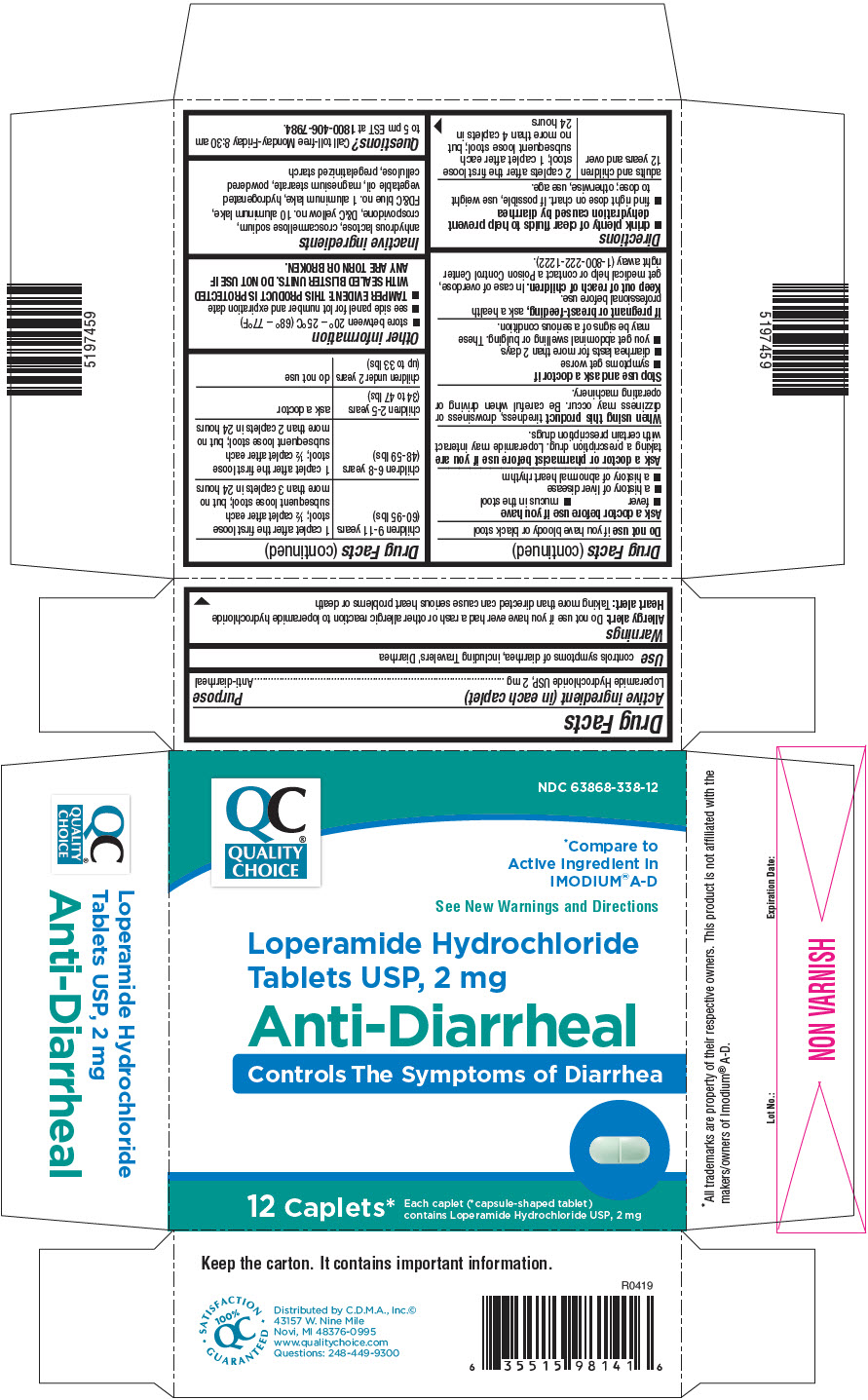
- Active ingredient (in each caplet)
- Purpose
- Use
-
Warnings
Allergy alert
Do not use if you have ever had a rash or other allergic reaction to loperamide hydrochloride
Ask a doctor before use if you have
- fever
- mucus in the stool
- a history of liver disease
- a history of abnormal heart rhythm
Ask a doctor or pharmacist before use if you aretaking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this producttiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
-
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
adults and children 12 years and over 2 caplets after the first loose stool; 1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours children 9-11 years
(60-95 lbs)1 caplet after the first loose stool; ½ caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours children 6-8 years
(48-59 lbs)1 caplet after the first loose stool; ½ caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours children 2-5 years
(34 to 47 lbs)ask a doctor children under 2 years
(up to 33 lbs)do not use - Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 2 mg Caplet Blister Pack Carton
-
INGREDIENTS AND APPEARANCE
LOPERAMIDE HYDROCHLORIDE
loperamide hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-338 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOPERAMIDE HYDROCHLORIDE (UNII: 77TI35393C) (LOPERAMIDE - UNII:6X9OC3H4II) LOPERAMIDE HYDROCHLORIDE 2 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYDROGENATED COTTONSEED OIL (UNII: Z82Y2C65EA) MAGNESIUM STEARATE (UNII: 70097M6I30) POWDERED CELLULOSE (UNII: SMD1X3XO9M) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color green Score 2 pieces Shape CAPSULE Size 9mm Flavor Imprint Code 123 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-338-24 24 in 1 BLISTER PACK; Type 0: Not a Combination Product 02/01/1993 2 NDC:63868-338-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/1993 3 NDC:63868-338-12 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 02/01/1993 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074091 02/01/1993 Labeler - Chain Drug Marketing Association Inc. (011920774) Registrant - Ranbaxy Pharmaceuticals Inc. (937890044) Establishment Name Address ID/FEI Business Operations Ohm Laboratories Inc. 051565745 manufacture(63868-338)