Label: TEETH CLEANING tablet, chewable
- NDC Code(s): 84732-026-01
- Packager: Dongguan Haiyi Technology Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Inactive ingredient
- Purpose section
-
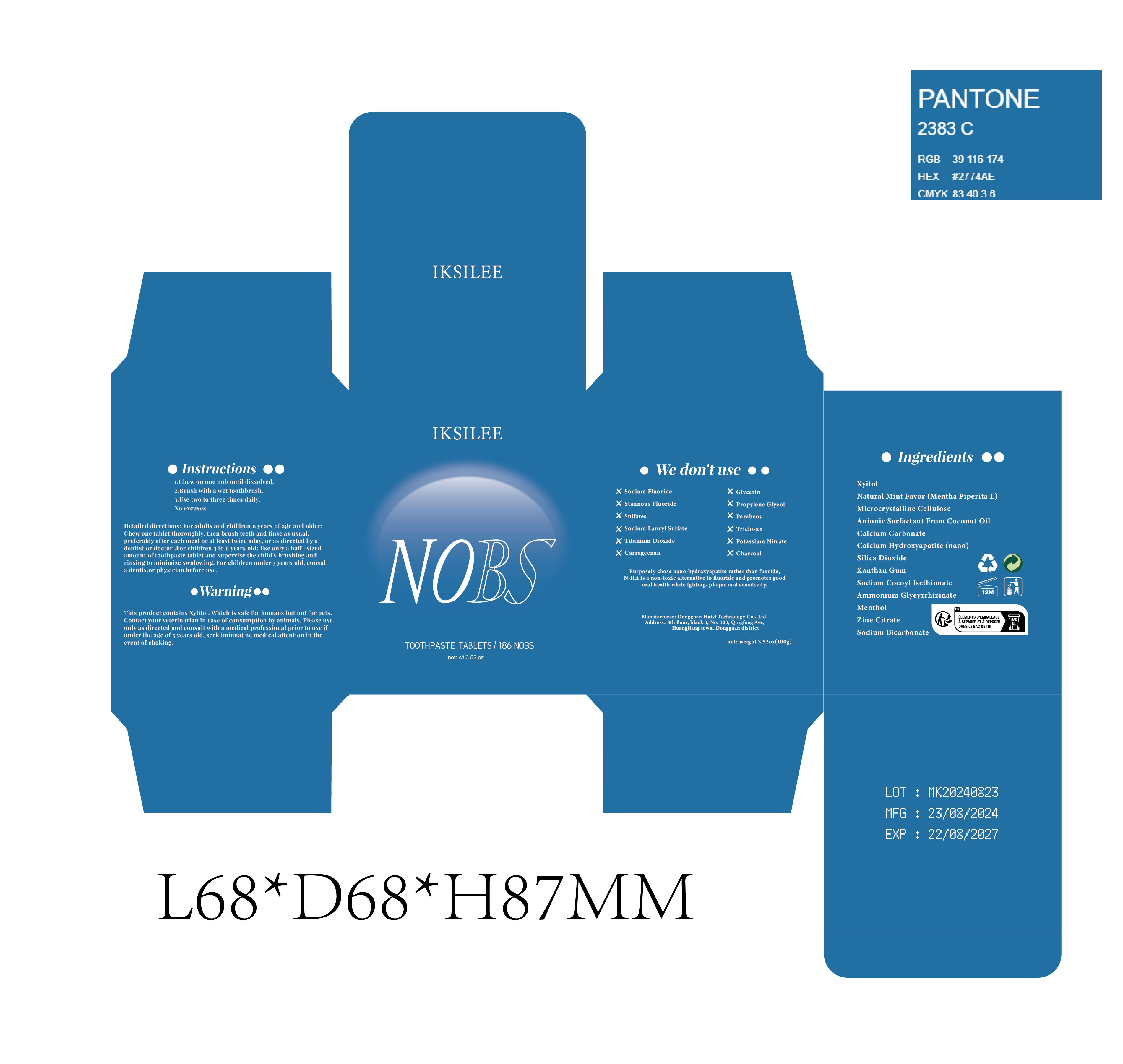
Warning
This produet contains Xylitol, Which is safe for humans but not for pets.Contact your veterinarian in case of consumption by animals, Please useonly as direeted and consult with a medical professional prior to use ifunder the age of 3 years old, seek iminnat ne medical attention in the event of choking.
-
stop use
1.Allergic reaction: If skin redness, itching or other allergic symptoms occur, stop using immediately and consult a doctor.
2. Oral irritation: If irritation or discomfort in the mouth or throat occurs after use, stop using.
3. Oral ulcers or wounds: If there are ulcers, wounds or other conditions in the mouth, and you feel uncomfortable after use, it is recommended to stop using and consult a doctor. Use by children: If swallowed by mistake or used by children, please stop using immediately and seek medical help - not use
- OUT OF CHILDREN
- HOW TO USE
- Dosage
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TEETH CLEANING
teeth cleaning tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84732-026 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength XYLITOL (UNII: VCQ006KQ1E) (XYLITOL - UNII:VCQ006KQ1E) XYLITOL 1 mg in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 mg in 100 g Inactive Ingredients Ingredient Name Strength ZINC CITRATE (UNII: K72I3DEX9B) AMMONIUM GLYCYRRHIZINATE TRIHYDRATE (UNII: 78NEL3149I) SODIUM BICARBONATE (UNII: 8MDF5V39QO) MINT (UNII: FV98Z8GITP) XANTHAN GUM (UNII: TTV12P4NEE) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) CALCIUM CARBONATE (UNII: H0G9379FGK) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white Score score with uneven pieces Shape ROUND Size 36992mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84732-026-01 100 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 09/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 09/29/2024 Labeler - Dongguan Haiyi Technology Co.,Ltd. (722030807) Establishment Name Address ID/FEI Business Operations Dongguan Haiyi Technology Co.,Ltd. 722030807 manufacture(84732-026)