Label: TETROXY HCA- oxytetracycline hcl powder
- NDC Code(s): 61133-5010-1, 61133-5010-2, 61133-5010-3
- Packager: Bimeda, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONTetroxy® HCA-1400 - (oxytetracycline hydrochloride soluble powder) Antibiotic - CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian. This packet ...
-
DOSAGE & ADMINISTRATION
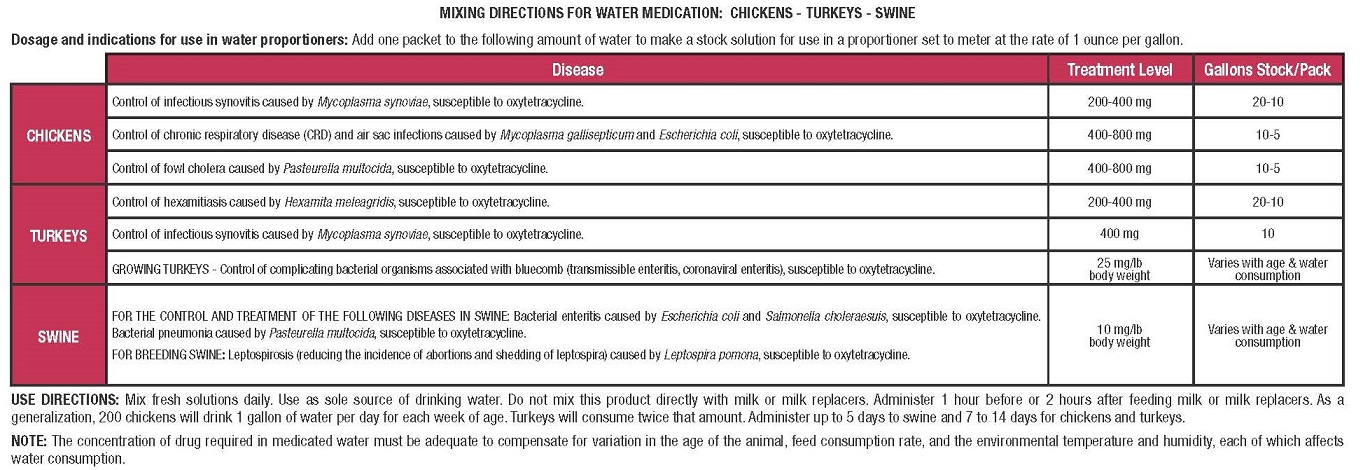
-
PRECAUTIONSCAUTION: Use as the sole source of oxytetracycline. Not to be used for more than 5 consecutive days in swine or 14 consecutive days in chickens and turkeys.
-
RESIDUE WARNINGWARNING: Do not feed to birds producing eggs for human consumption.
-
STORAGE AND HANDLINGRECOMMENDED PACKET STORAGE CONDITIONS: Store between 20°C - 25°C (68°F - 77°F) with excursions permitted between 15°C - 40°C (59°F - 104°F). CONTACT INFORMATION: To report adverse events, for ...
-
PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information



