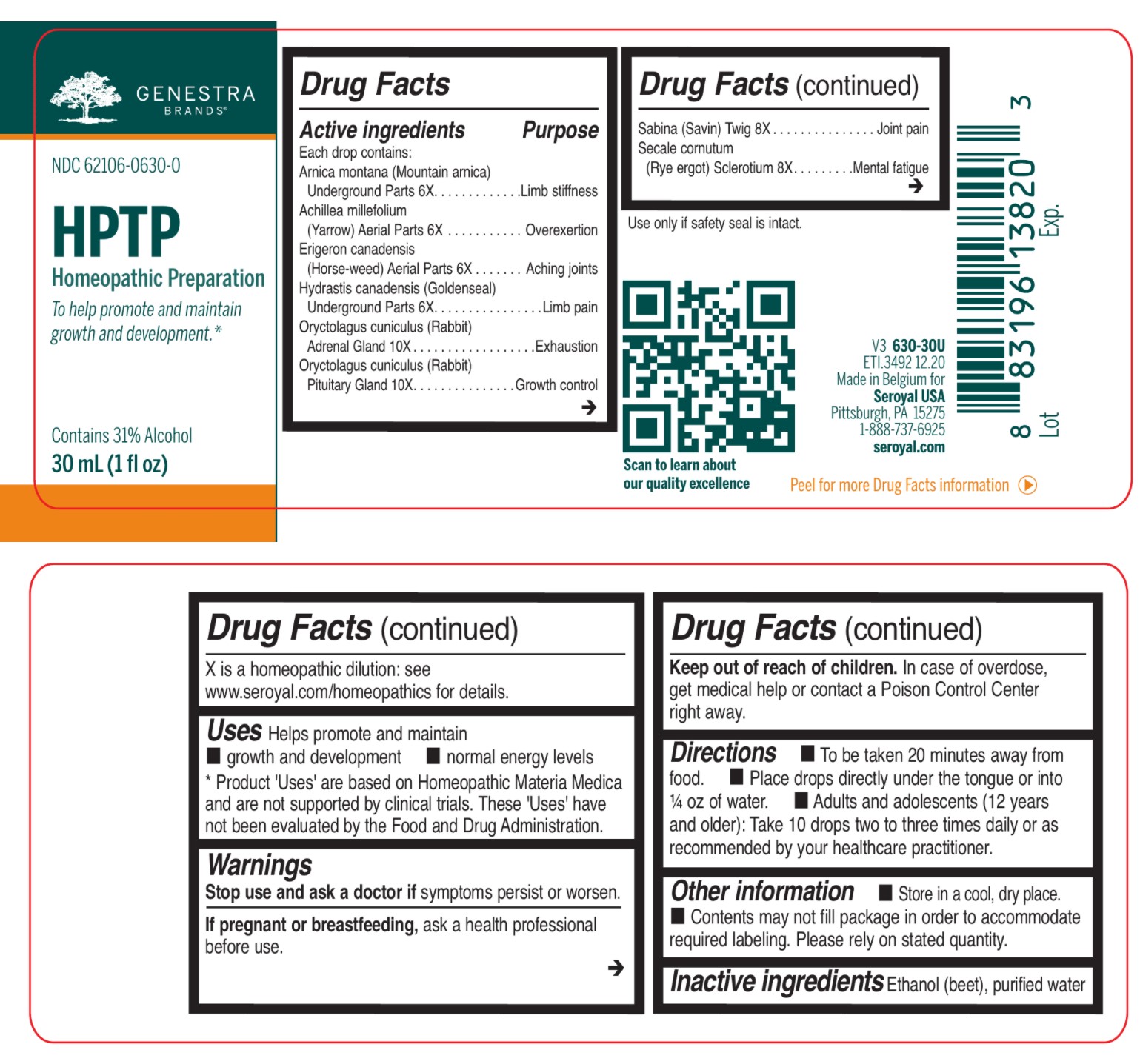
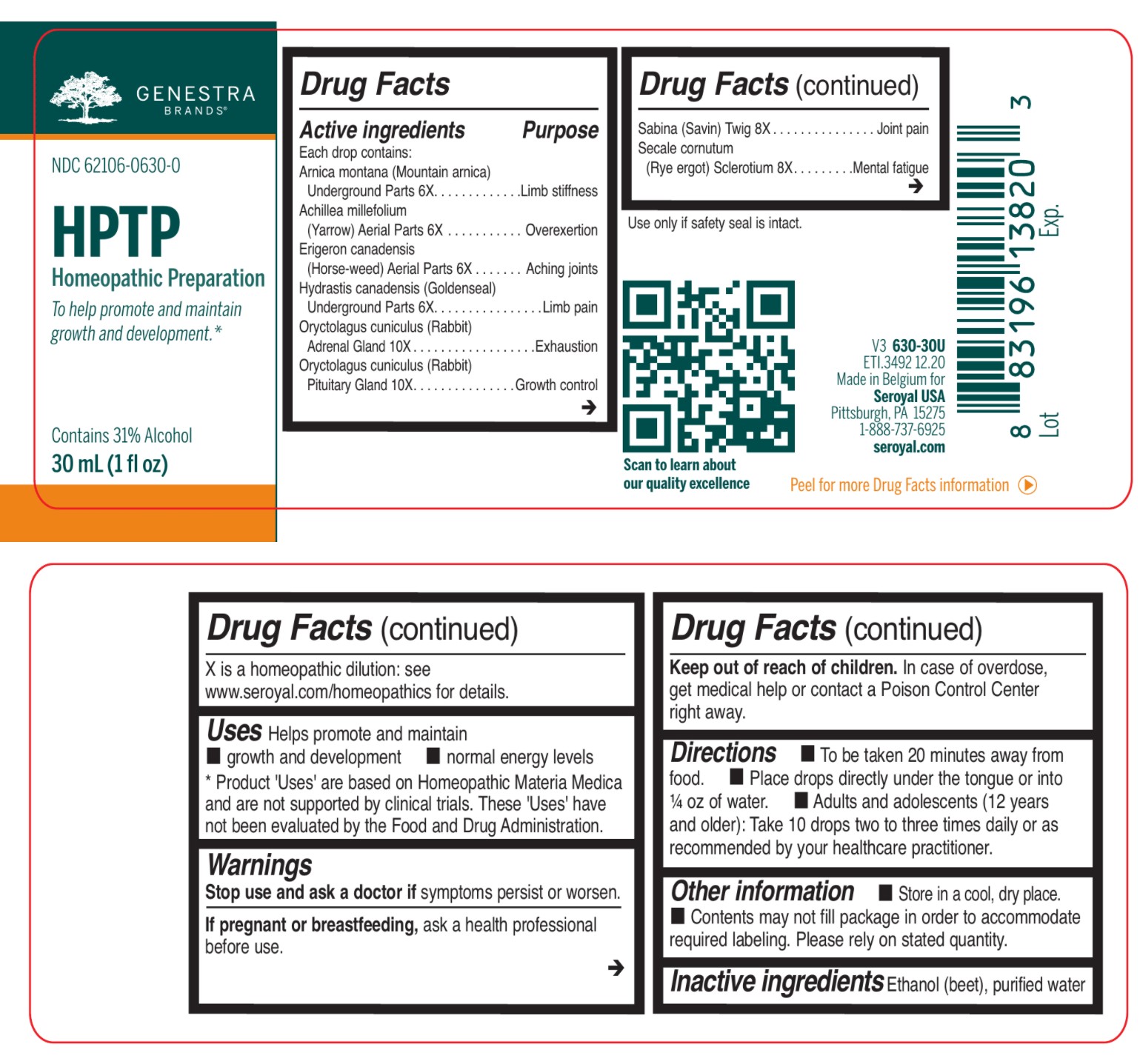
Label: HPTP- arnica montana, achillea millefolium, erigeron canadensis, hydrastis canadensis, oryctolagus cuniculus, sabina, secale cornutum liquid
- NDC Code(s): 62106-0630-0
- Packager: Seroyal USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 22, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Arnica montana (Mountain arnica) Underground Parts 6X
Achillea millefolium (Yarrow) Aerial Parts 6X
Erigeron canadensis (Horse-weed) Aerial Parts 6X
Hydrastis canadensis (Goldenseal) Underground Parts 6X
Oryctolagus cuniculus (Rabbit) Adrenal Gland 10X
Oryctolagus cuniculus (Rabbit) Pituitary Gland 10XSabina (Savin) Twig 8X
Secale cornutum (Rye ergot) Sclerotium 8X - PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
-
DOSAGE & ADMINISTRATION
Directions
To be taken 20 minutes away from food.
Place drops directly under the tongue or into ¼ oz of water.
Adults and adolescents (12 years and older): Take 10 drops two to three times daily or
as recommended by your healthcare practitioner.Children (under 12 years): Take under the direction of your healthcare practitioner.
-
INDICATIONS & USAGE
Uses
Helps promote and maintain growth and development normal energy levels.
Directions
To be taken 20 minutes away from food.
Place drops directly under the tongue or into ¼ oz of water.
Adults and adolescents (12 years and older): Take 10 drops two to three times daily or
as recommended by your healthcare practitioner.Children (under 12 years): Take under the direction of your healthcare practitioner.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HPTP
arnica montana, achillea millefolium, erigeron canadensis, hydrastis canadensis, oryctolagus cuniculus, sabina, secale cornutum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-0630 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength JUNIPERUS SABINA LEAFY TWIG (UNII: Z5BEX9K2G1) (JUNIPERUS SABINA LEAFY TWIG - UNII:Z5BEX9K2G1) JUNIPERUS SABINA LEAFY TWIG 8 [hp_X] in 30 mL CLAVICEPS PURPUREA SCLEROTIUM (UNII: 01G9XEA93N) (CLAVICEPS PURPUREA SCLEROTIUM - UNII:01G9XEA93N) CLAVICEPS PURPUREA SCLEROTIUM 8 [hp_X] in 30 mL ARNICA MONTANA ROOT (UNII: MUE8Y11327) (ARNICA MONTANA ROOT - UNII:MUE8Y11327) ARNICA MONTANA ROOT 6 [hp_X] in 30 mL ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 6 [hp_X] in 30 mL CONYZA CANADENSIS (UNII: 16D08B0B9N) (CONYZA CANADENSIS - UNII:16D08B0B9N) CONYZA CANADENSIS 6 [hp_X] in 30 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 6 [hp_X] in 30 mL ORYCTOLAGUS CUNICULUS PITUITARY GLAND (UNII: T9F9ZW799A) (ORYCTOLAGUS CUNICULUS PITUITARY GLAND - UNII:T9F9ZW799A) ORYCTOLAGUS CUNICULUS PITUITARY GLAND 10 [hp_X] in 30 mL ORYCTOLAGUS CUNICULUS ADRENAL GLAND (UNII: QGY289M89Q) (ORYCTOLAGUS CUNICULUS ADRENAL GLAND - UNII:QGY289M89Q) ORYCTOLAGUS CUNICULUS ADRENAL GLAND 10 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-0630-0 1 in 1 CARTON 10/26/2015 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/26/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN'UP 401010287 manufacture(62106-0630)