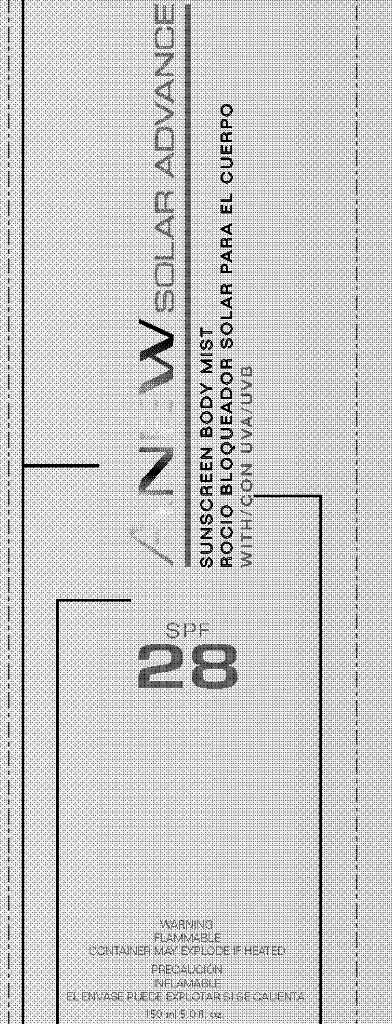
Label: ANEW SOLAR ADVANCE SUNSCREEN BODY MIST- homosalate, octinoxate, octisalate, oxybenzone, avobenzone, octocrylene spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 10096-0254-1, 10096-0254-2 - Packager: Avon Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 11, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- WARNINGS
- INDICATIONS & USAGE
- SAFE HANDLING WARNING
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
OTHER INGREDIENTS/OTROS INGREDIENTES: SD
ALCOHOL 40-B, C12-15 ALKYL BENZOATE, PPG-2
MYRISTYL ETHER PROPIONATE, DIMETHICONE, GLYCERIN,
ACRYLATES/OCTYLACRYLAMIDE COPOLYMER,
POLYESTER-8, DILAURYL THIODIPROPIONATE,
KAEMPFERIA GALANGA ROOT EXTRACT, GLYCINE SOJA
(SOYBEAN) SEED EXTRACT, PHAEODACTYLUM
TRICORNUTUM EXTRACT, ORYZANOL, FOENICULUM
VULGARE (FENNEL) FRUIT EXTRACT, DAUCUS CAROTA
SATIVA (CARROT) ROOT EXTRACT, TOCOPHEROL,
CAPRYLIC/CAPRIC TRIGLYCERIDE, CAPRYLYL GLYCOL,
PARFUM/ FRAGRANCE, WATER/EAU. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANEW SOLAR ADVANCE SUNSCREEN BODY MIST
homosalate, octinoxate, octisalate, oxybenzone, avobenzone, octocrylene sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10096-0254 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 12 mL in 150 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 11.25 mL in 150 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 7.125 mL in 150 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 7.125 mL in 150 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 4.275 mL in 150 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 3.84 mL in 150 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10096-0254-2 1 in 1 CARTON 1 NDC:10096-0254-1 150 mL in 1 CAN Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/11/2011 Labeler - Avon Products, Inc. (001468693)