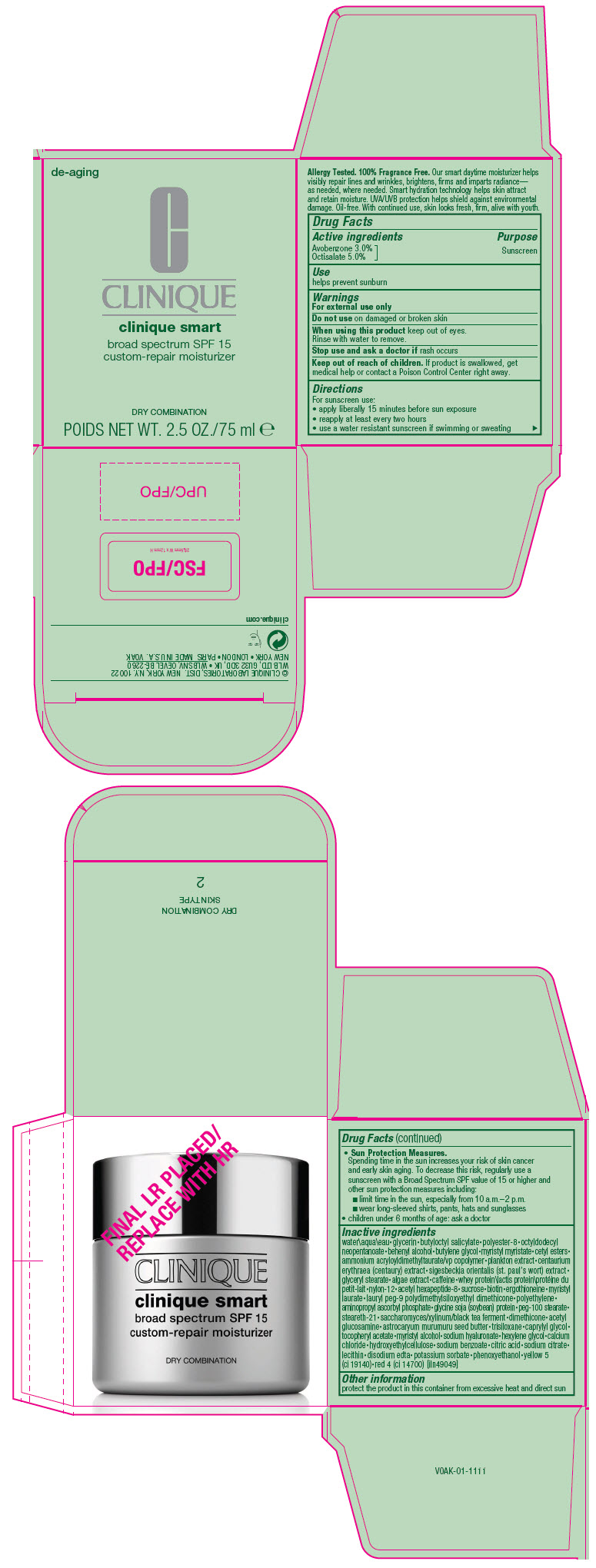
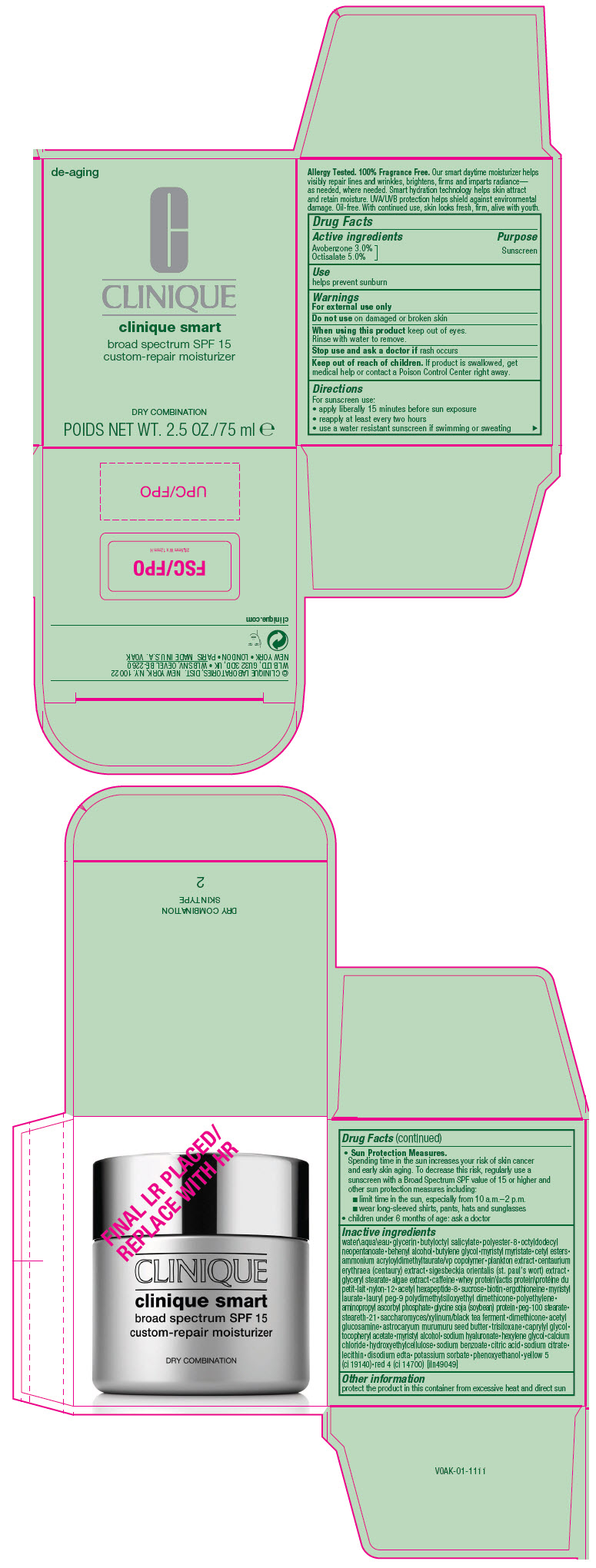
Label: CLINIQUE SMART BROAD SPECTRUM SPF 15 CUSTOM-REPAIR MOISTURIZER DRY COMBINATION- avobenzone and octisalate cream
-
NDC Code(s):
49527-051-01,
49527-051-02,
49527-051-03,
49527-051-04, view more49527-051-05
- Packager: CLINIQUE LABORATORIES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqua\eau • glycerin • butyloctyl salicylate • polyester-8 • octyldodecyl neopentanoate • behenyl alcohol • butylene glycol • myristyl myristate • cetyl esters • ammonium acryloyldimethyltaurate/vp copolymer • plankton extract • centaurium erythraea (centaury) extract • sigesbeckia orientalis (st. paul's wort) extract • glyceryl stearate • algae extract • caffeine • whey protein\lactis protein\protéine du petit-lait • nylon-12 • acetyl hexapeptide-8 • sucrose • biotin • ergothioneine • myristyl laurate • lauryl peg-9 polydimethylsiloxyethyl dimethicone • polyethylene • aminopropyl ascorbyl phosphate • glycine soja (soybean) protein • peg-100 stearate • steareth-21 • saccharomyces/xylinum/black tea ferment • dimethicone • acetyl glucosamine • astrocaryum murumuru seed butter • trisiloxane • caprylyl glycol • tocopheryl acetate • myristyl alcohol • sodium hyaluronate • hexylene glycol • calcium chloride • hydroxyethylcellulose • sodium benzoate • citric acid • sodium citrate • lecithin • disodium edta • potassium sorbate • phenoxyethanol • yellow 5 (ci 19140) • red 4 (ci 14700) [iln49049]
- Other information
- PRINCIPAL DISPLAY PANEL - 75 ml Jar Carton
-
INGREDIENTS AND APPEARANCE
CLINIQUE SMART BROAD SPECTRUM SPF 15 CUSTOM-REPAIR MOISTURIZER DRY COMBINATION
avobenzone and octisalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-051 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) CETYL ESTERS WAX (UNII: D072FFP9GU) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) BLUEFISH (UNII: 79U8P8X3Q5) CENTAURIUM ERYTHRAEA (UNII: 57X4TSH58S) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CAFFEINE (UNII: 3G6A5W338E) NYLON-12 (UNII: 446U8J075B) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) SUCROSE (UNII: C151H8M554) BIOTIN (UNII: 6SO6U10H04) ERGOTHIONEINE (UNII: BDZ3DQM98W) MYRISTYL LAURATE (UNII: 58U0NZN2BT) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) AMINOPROPYL ASCORBYL PHOSPHATE (UNII: 290O2PQ83R) SOYBEAN (UNII: L7HT8F1ZOD) PEG-100 STEARATE (UNII: YD01N1999R) STEARETH-21 (UNII: 53J3F32P58) DIMETHICONE (UNII: 92RU3N3Y1O) N-ACETYLGLUCOSAMINE (UNII: V956696549) ASTROCARYUM MURUMURU SEED BUTTER (UNII: 12V64UPU6R) TRISILOXANE (UNII: 9G1ZW13R0G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MYRISTYL ALCOHOL (UNII: V42034O9PU) HYALURONATE SODIUM (UNII: YSE9PPT4TH) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PHENOXYETHANOL (UNII: HIE492ZZ3T) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 4 (UNII: X3W0AM1JLX) DOCOSANOL (UNII: 9G1OE216XY) SIGESBECKIA ORIENTALIS WHOLE (UNII: ZM9Q0FEI4Z) AGAR, UNSPECIFIED (UNII: 89T13OHQ2B) WHEY PROTEIN HYDROLYSATE (UNII: 237DZG2JLA) SACCHAROMYCES CEREVISIAE (UNII: 978D8U419H) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) CITRIC ACID ACETATE (UNII: DSO12WL7AU) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-051-01 1 in 1 CARTON 10/01/2015 1 50 mL in 1 JAR; Type 0: Not a Combination Product 2 NDC:49527-051-02 1 in 1 CARTON 07/12/2022 2 75 mL in 1 JAR; Type 0: Not a Combination Product 3 NDC:49527-051-03 1 in 1 CARTON 07/12/2022 3 15 mL in 1 JAR; Type 0: Not a Combination Product 4 NDC:49527-051-04 7 mL in 1 PACKET; Type 0: Not a Combination Product 07/12/2022 10/05/2023 5 NDC:49527-051-05 1 in 1 CARTON 10/01/2015 5 7 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2015 Labeler - CLINIQUE LABORATORIES LLC (044475127) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd. 202952982 manufacture(49527-051) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd. 204132062 pack(49527-051) , label(49527-051) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(49527-051) , pack(49527-051) , label(49527-051) Establishment Name Address ID/FEI Business Operations Northtec LLC 943871157 pack(49527-051) , label(49527-051) Establishment Name Address ID/FEI Business Operations NORTHTEC KEYSTONE 949264774 pack(49527-051) , label(49527-051) Establishment Name Address ID/FEI Business Operations PALC 078364654 pack(49527-051) , label(49527-051)