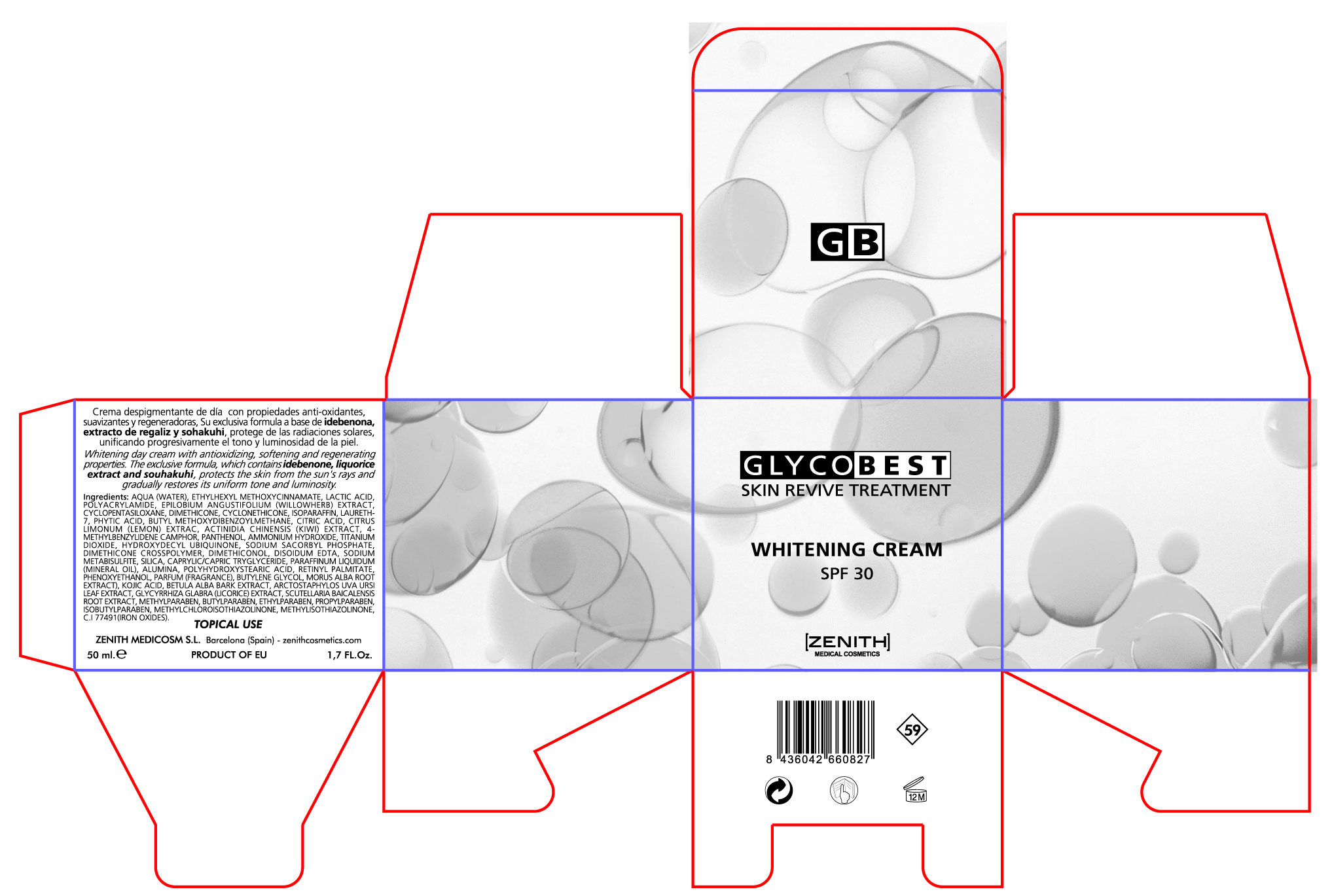
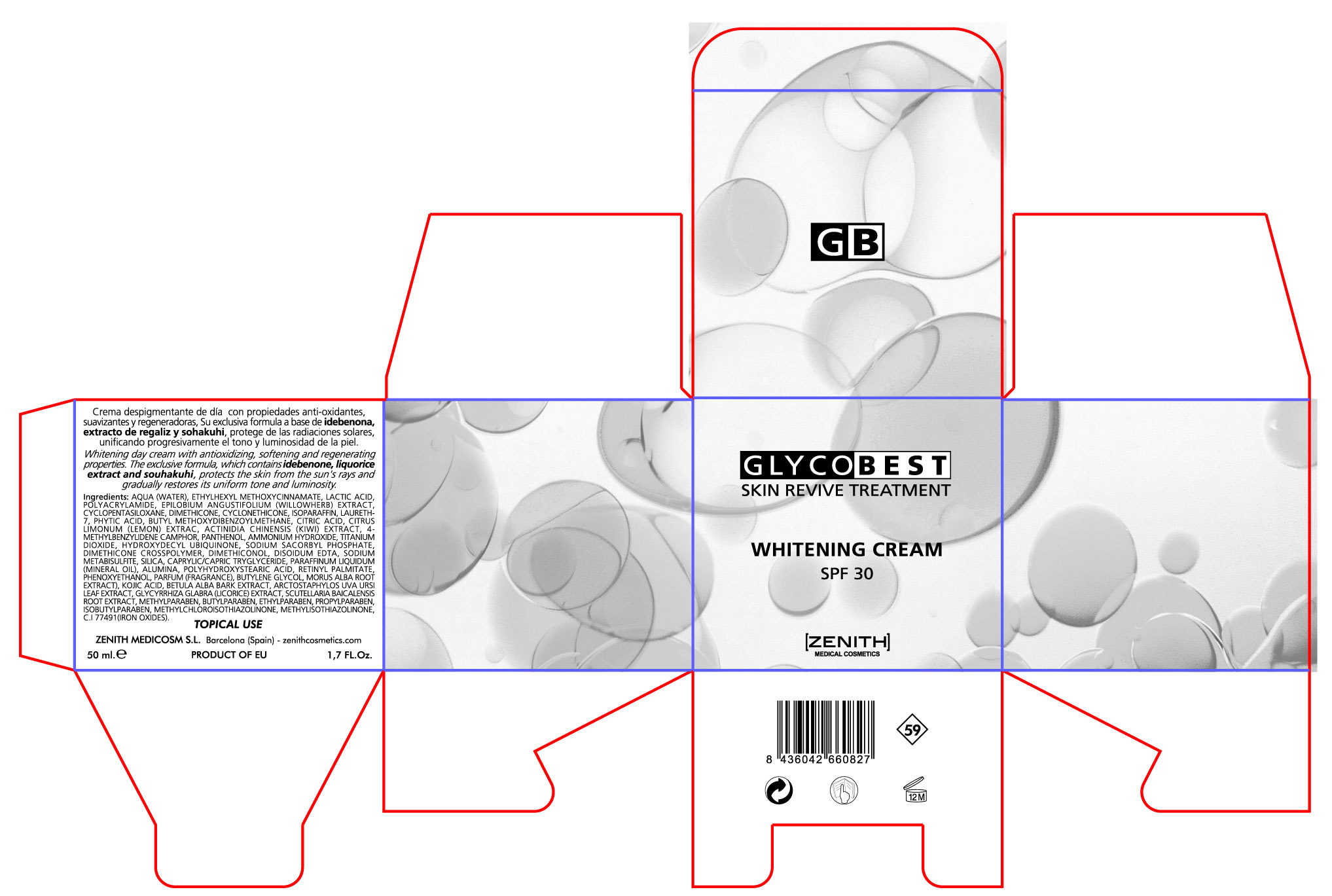
Label: WHITENING GLYCOBEST- octinoxate avobenzone titanium oxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 42248-129-02 - Packager: Zenith Medicosm SL
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 23, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
-
Description
A cream with high concentrations of Vitamin A, Vitamin C and marine collagen, which are particularly indicated for oily and aging skin. It combines the regenerating action of Retinol and the bateriostatic action of Vitamin C to fight premature skin aging. Softens and smoothes the skin, reduces wrinkles and gives the skin an even, radiant glow.
200ml. 6.8Fl.Oz.
- Warning
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WHITENING GLYCOBEST
octinoxate avobenzone titanium oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42248-129 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.5 mL in 50 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1.5 mL in 50 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 12.5 mL in 50 mL Inactive Ingredients Ingredient Name Strength LACTIC ACID (UNII: 33X04XA5AT) PROPYLPARABEN (UNII: Z8IX2SC1OH) POLYACRYLAMIDE (1500 MW) (UNII: 5D6TC4BRWV) CYCLOMETHICONE (UNII: NMQ347994Z) ARCTOSTAPHYLOS UVA-URSI LEAF (UNII: 3M5V3D1X36) LICORICE (UNII: 61ZBX54883) WATER (UNII: 059QF0KO0R) EPILOBIUM ANGUSTIFOLIUM LEAF (UNII: 7NV86426N2) LEMON JUICE (UNII: AGN709ANTJ) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) LAURETH-7 (UNII: Z95S6G8201) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) fytic acid (UNII: 7IGF0S7R8I) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) METHYLPARABEN (UNII: A2I8C7HI9T) ENZACAMENE (UNII: 8I3XWY40L9) BETULA PUBESCENS BARK (UNII: 3R504894L9) TRICLOSAN (UNII: 4NM5039Y5X) EDETATE DISODIUM (UNII: 7FLD91C86K) DIMETHICONOL (41 MPA.S) (UNII: 343C7U75XW) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ETHYLPARABEN (UNII: 14255EXE39) BUTYLPARABEN (UNII: 3QPI1U3FV8) iSOBUTYLPARABEN (UNII: 0QQJ25X58G) PANTHENOL (UNII: WV9CM0O67Z) WHITE MULBERRY (UNII: MN25R0HH5A) SODIUM METABISULFITE (UNII: 4VON5FNS3C) IDEBENONE (UNII: HB6PN45W4J) TRICAPRYLIN (UNII: 6P92858988) KOJIC ACID (UNII: 6K23F1TT52) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42248-129-02 1 in 1 BOX 1 50 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 08/15/2011 Labeler - Zenith Medicosm SL (464239694) Registrant - Zenith Medicosm SL (464239694) Establishment Name Address ID/FEI Business Operations Zenith Medicosm SL 464239694 manufacture