Label: ADZYNMA- apadamtase alfa kit
-
NDC Code(s):
64764-130-01,
64764-135-01,
64764-140-05,
64764-145-05, view more64764-515-50
- Packager: TAKEDA PHARMACEUTICALS AMERICA, INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated August 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ADZYNMA safely and effectively. See full prescribing information for ADZYNMA.
ADZYNMA (ADAMTS13, recombinant-krhn)
lyophilized powder for Injection, for intravenous use
Initial U.S. Approval: 2023INDICATIONS AND USAGE
ADZYNMA (ADAMTS13, recombinant-krhn) is a human recombinant “A disintegrin and metalloproteinase with thrombospondin motifs 13” (rADAMTS13) indicated for prophylactic or on demand enzyme replacement therapy (ERT) in adult and pediatric patients with congenital thrombotic thrombocytopenic purpura (cTTP). (1)
DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only. (2)
Prophylactic Therapy
- Administer 40 IU/kg body weight once every other week intravenously at a rate of 2 to 4 mL per minute. (2.1)
- The prophylaxis dosing frequency may be adjusted to 40 IU/kg body weight once weekly based on prior prophylactic dosing regimen or clinical response. (2.1)
On-Demand Therapy
Administer intravenously at a rate of 2 to 4 mL per minute:
DOSAGE FORMS AND STRENGTHS
ADZYNMA is available as a lyophilized powder in single-dose vials containing nominally 500 or 1500 international units. (3)
CONTRAINDICATIONS
Do not use in patients who have manifested life threatening hypersensitivity reactions to ADZYNMA or its components. (4)
WARNINGS AND PRECAUTIONS
Hypersensitivity reactions may occur. Discontinue ADZYNMA if hypersensitivity symptoms occur and administer appropriate emergency treatment. (5.1)
Immunogenicity: Patients may develop antibodies to rADAMTS13 which could potentially result in a decreased or lack of response to rADAMTS13. Patients may develop antibodies to host cell proteins which could potentially result in adverse reactions. There are no data on risk in previously untreated patients (subjects naïve to plasma-based products). (5.2)
ADVERSE REACTIONS
Most common adverse reactions (incidence >5%) are headache, diarrhea, migraine, abdominal pain, nausea, upper respiratory tract infection, dizziness, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Immunogenicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
ADZYNMA (ADAMTS13, recombinant-krhn) is a human recombinant “A disintegrin and metalloproteinase with thrombospondin motifs 13” (rADAMTS13) indicated for prophylactic or on demand enzyme replacement therapy (ERT) in adult and pediatric patients with congenital thrombotic thrombocytopenic purpura (cTTP) [see Use in Specific Populations (8.4), Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
2.1 Dosage
- Each vial of ADZYNMA is labeled with the actual rADAMTS13 activity, measured in terms of its potency in International Units (IU).
- Calculate administration dose and volume based on the patient's body weight using the actual potency (and not the nominal potency) as printed on ADZYNMA vial.
- For Intravenous (IV) Infusion at a rate of 2 to 4 mL per minute.
Prophylactic Therapy
The recommended prophylactic dosage regimen of ADZYNMA is as follows:
- Administer 40 IU/kg body weight once every other week.
- The prophylactic dosing frequency may be adjusted to 40 IU/kg body weight once weekly based on prior prophylactic dosing regimen or clinical response [see Use in Specific Populations (8.4), Clinical Pharmacology (12.3), Clinical Studies (14)].
On-Demand Therapy
A guide for dosing ADZYNMA for on demand treatment of an acute event is provided in Table 1.
Table 1: Dosing for On-Demand Therapy Treatment Day 1 Treatment Day 2 Treatment Day 3 and Beyond 40 IU/kg 20 IU/kg 15 IU/kg once daily until two days after the acute event is resolved. 2.2 Preparation and Administration
- Use aseptic technique (clean and germ-free) throughout the procedure.
- Check the expiration date of the product prior to use.
- Do not use ADZYNMA if the expiration date has passed.
Reconstitution
- 1.
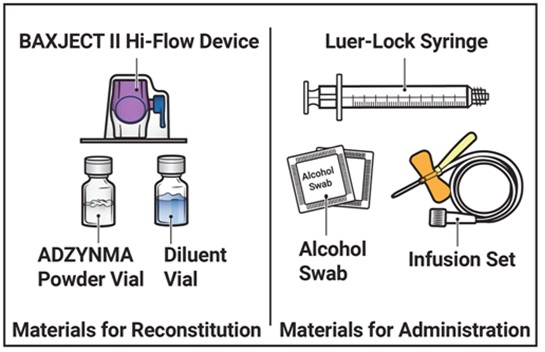
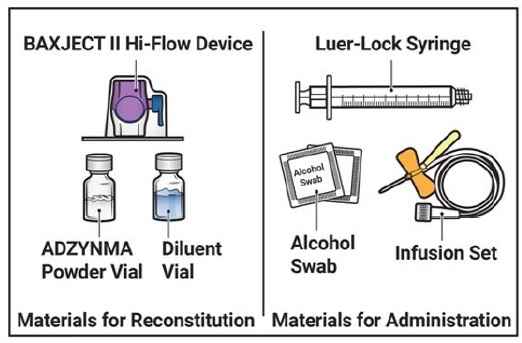
- Prepare a clean, germ-free, flat surface and gather all the materials you will need for the reconstitution and infusion. Figure A depicts the materials provided in the carton box.
Figure A
- 2.
-
Do not use ADZYNMA if the expiration date has passed.
- Use ADZYNMA within 3 hours after reconstitution and keep at room temperature not to exceed 86°F/30°C. Do not store at any other temperature. Discard any unused reconstituted product if not used within 3 hours after reconstitution.
- 3.
- Allow the vials of ADZYNMA and diluent to reach room temperature before use.
- If the patient needs more than one vial of ADZYNMA per injection, reconstitute each vial according to the instructions stated under ‘Reconstitution’.
- Inspect the reconstituted ADZYNMA solution for particulate matter and discoloration prior to administration. The solution should be clear and colorless in appearance.
- Do not administer if particulate matter or discoloration is observed.
- 4.
- Wash and dry your hands thoroughly, and put on clean exam gloves.
- 5.
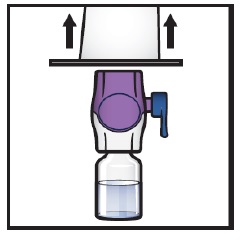
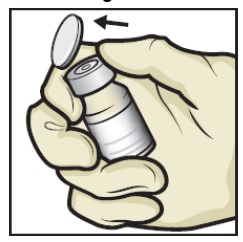
- Remove plastic caps from the ADZYNMA and diluent vials and place the vials on a flat surface (Figure B).
Figure B
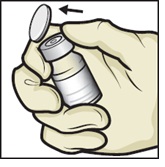
- 6.
- Wipe the rubber stoppers with an alcohol swab and allow them to dry prior to use (Figure C).
Figure C
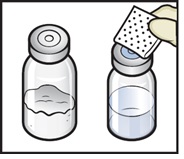
- 7.
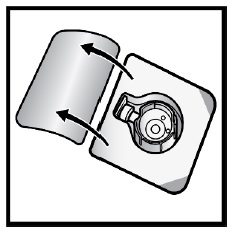
- Open the BAXJECT II Hi-Flow device package by peeling away the lid, without touching the inside (Figure D).
- Do not remove the BAXJECT II Hi-Flow device from the package.
- Do not touch the clear plastic spike.
Figure D
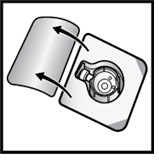
- 8.
- Turn the package with the BAXJECT II Hi-Flow device upside down and place it over the top of the diluent vial. Press straight down until the clear plastic spike pierces through the diluent vial stopper (Figure E).
Figure E
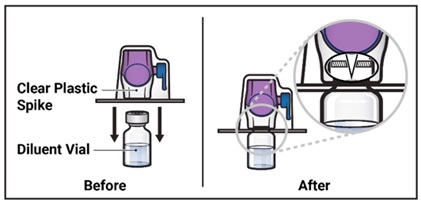
- 9.
- Grip the BAXJECT II Hi-Flow device package at its edge and pull the package off the device (Figure F).
- Do not remove the blue cap from the BAXJECT II Hi-Flow device.
- Do not touch the exposed purple plastic spike.
Figure F
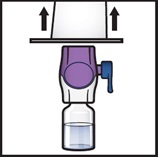
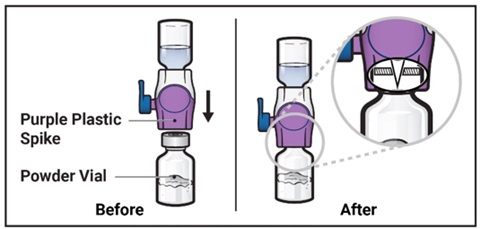
- 10.
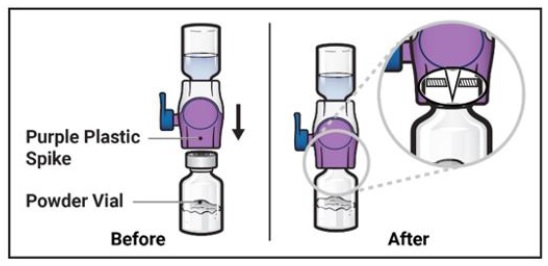
-
Turn the system over so that the diluent vial is now on top. Press the BAXJECT II Hi-Flow device straight down until the purple plastic spike pierces through the ADZYNMA powder vial stopper (Figure G). The vacuum will draw the diluent into the ADZYNMA powder vial.
- You may notice some bubbles or foam – this is normal and should soon disappear. Wait until foam or bubbles dissipate before administration.
Figure G
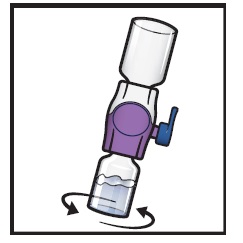
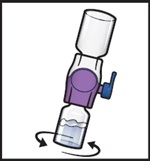
- 11.
- Swirl the connected vials gently and continuously until the powder is completely dissolved (Figure H).
- Do not shake the vial.
Figure H
- 12.
- Visually inspect the reconstituted solution for particulate matter before administration. The solution should be clear and colorless in appearance.
- Do not use the product if particulate matter or discoloration is observed.
- 13.
- If the dose requires more than one vial of ADZYNMA, repeat step 1 to step 12 to reconstitute each vial.
- Use a different BAXJECT II Hi-Flow device to reconstitute each vial of ADZYNMA and diluent.
- 14.
- Take off the blue cap from the BAXJECT II Hi-Flow device (Figure I). Attach a Luer-lock syringe (Figure J).
- Do not inject air into the system.
Figure I
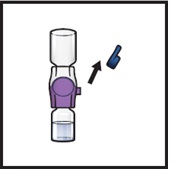
Figure J
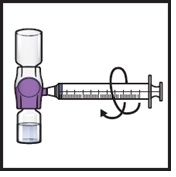
- 15.
-
Turn the system upside down (ADZYNMA vial is now on top). Draw the reconstituted solution into the syringe by pulling the plunger back slowly (Figure K).
- Use ADZYNMA within 3 hours after reconstitution and keep at room temperature not to exceed 86°F/30°C. Do not store at any other temperature. Discard any unused reconstituted product if not used within 3 hours after reconstitution.
- Do not administer ADZYNMA in the same tubing or container at the same time with other medicinal products for infusion.
Figure K 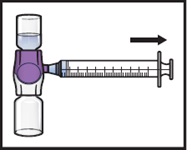
- 16.
- If a patient is to receive more than one vial of ADZYNMA, the contents of multiple vials can be drawn into the same syringe. Repeat this process for all reconstituted vials of ADZYNMA until the total volume to be administered is reached.
- 17.
- Disconnect the syringe and attach a suitable injection needle or an infusion set.
- 18.
- Point the needle up and remove any air bubbles by gently tapping the syringe with your finger and slowly and carefully pushing air out of the syringe and needle.
- 19.
- Apply a tourniquet and clean the chosen infusion site with an alcohol swab (Figure L).
Figure L 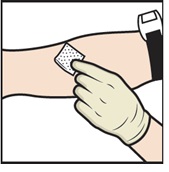
- 20.
- Insert the needle into the vein and remove the tourniquet.
- 21.
- Infuse the reconstituted ADZYNMA slowly, at a rate of 2 to 4 mL per minute (Figure M).
- A syringe pump may be used to control the rate of administration.
Figure M 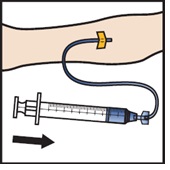
- 22.
- Take the needle out of the vein, place a cotton ball or gauze on the infusion site, and apply pressure for several minutes to stop bleeding.
- Do not recap the needle.
- 23.
- Place the needle, syringe, and empty vials in a puncture-resistant sharps container.
- Do not dispose of syringes and needles in the regular waste.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ADZYNMA is contraindicated in patients who have manifested life threatening hypersensitivity reactions to ADZYNMA or its components [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Allergic-type hypersensitivity including anaphylactic reactions may occur with ADZYNMA. Patients should be informed of the early signs of hypersensitivity including but not limited to tachycardia, tightness of the chest, wheezing and/or acute respiratory distress, hypotension, generalized urticaria, pruritus, rhinoconjunctivitis, angioedema, lethargy, nausea, vomiting, paresthesia, and restlessness. If signs and symptoms of severe allergic reactions occur, immediately discontinue administration of ADZYNMA and provide appropriate supportive care.
5.2 Immunogenicity
There is a potential for immunogenicity with ADZYNMA. Patients may develop neutralizing antibodies to ADAMTS13, which could potentially result in a decreased or lack of response to ADAMTS13. Neutralizing antibodies were not reported in the cTTP clinical trials. All subjects had been previously exposed to ADAMTS13 through plasma-based products. There are no data on immunogenicity with ADZYNMA in previously untreated patients (subjects naïve to plasma-based products) [see Clinical Pharmacology (12.6)].
Patients may develop antibodies to host cell proteins which could potentially result in adverse reactions. There are no data on immunogenicity to host cell proteins in previously untreated patients (subjects naïve to plasma-based products).
-
6 ADVERSE REACTIONS
Most common adverse reactions (>5% of subjects) reported in clinical trials were headache, diarrhea, migraine, abdominal pain, nausea, upper respiratory tract infection, dizziness, and vomiting.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety profile of ADZYNMA was evaluated in one, prospective, randomized, active-controlled, open-label, multicenter, two-period crossover study (Study 1). The adverse drug reactions (ADR) are listed in Table 2.
Table 2: Adverse Reactions Reported in >5% of Patients Treated with ADZYNMA Adverse Reaction ADZYNMA (N= 48)
n (%)*N = Total number of patients treated with ADZYNMA in Study 1.
n = Number of patients who had at least one event in the category.- *
- Percentages by patient were calculated using the number of all subjects who had the listed adverse event.
Headache 15 (31.3) Diarrhea 8 (16.7) Migraine 7 (14.6) Abdominal pain 6 (12.5) Nausea 6 (12.5) Upper respiratory tract infection 6 (12.5) Dizziness 5 (10.4) Vomiting 5 (10.4) -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The safety of ADZYNMA for use during pregnancy has not been established in controlled clinical trials. Limited data with ADZYNMA use during pregnancy are insufficient to inform a drug-associated risk of adverse developmental outcomes.1 In determining whether ADZYNMA should be used in pregnancy, healthcare providers should balance the potential benefits with the potential risks.
The background rate of major birth defects and miscarriage in the indicated population is unknown. In the U.S. general population, the estimated background rate of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
There have been four cTTP patients exposed to ADZYNMA during pregnancy.
Two patients in a long-term extension study were found to be pregnant early in the first trimester while receiving prophylaxis with ADZYNMA. Both patients were discontinued from the study to comply with the protocol requirements. The first patient had no further exposure to ADZYNMA and had a first trimester miscarriage approximately two months after study discontinuation. The investigator assessed the event was unrelated to ADZYNMA. The second patient resumed treatment with ADZYNMA under a compassionate use program and delivered a healthy full-term baby with no safety concerns reported by the investigator.
Two additional cTTP patients were treated with ADZYNMA in a compassionate use program during pregnancy. The first patient, in the third trimester of her second pregnancy, experienced a stroke and thrombocytopenia that was refractory to daily plasmapheresis. At 33 weeks of gestation, ADZYNMA treatment was started once weekly. ADAMTS13 activity levels normalized, thrombocytopenia resolved, and a healthy baby was delivered at 37 weeks with no safety concerns reported by the treating physician due to ADZYNMA.1 The second patient had an exacerbation of her cTTP during her second trimester of pregnancy despite prior daily plasma exchange. Her pregnancy was considered to be at risk, with inadequate response to plasma-based therapies. ADZYNMA was started once weekly and induced clinical remission. The baby was delivered by a cesarean section at week 29 and the treating physician reported no adverse events due to ADZYNMA.
Whether ADZYNMA should be used in pregnancy is solely up to the medical judgment of the healthcare provider.
Nonclinical Data
In an embryo-fetal development study in rats, ADZYNMA was administered in female rats at 80, 200, or 400 IU/kg [up to 10x human equivalent dose (HED)] every third day from 2 weeks before mating through Day 16 of gestation and was not associated with any adverse maternal findings or treatment-related effects on embryo-fetal development. In a pre- and post-natal development study in rats, ADZYNMA was administered at 80, 200, or 400 IU/kg [up to 10x HED] by intravenous (IV) bolus injection every three days from Day 6 of gestation to weaning at approximately Day 21 of lactation. There were no adverse maternal effects or findings in F1 or F2 offspring.
8.2 Lactation
Risk Summary
There is no information regarding the presence of ADZYNMA in human milk, its effects on milk production or the breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ADZYNMA and any potential adverse effects to the breastfed child.
8.4 Pediatric Use
The safety and effectiveness of ADZYNMA has been established in pediatric patients. Clinical trials included patients aged 2 years and older. Based on data from population pharmacokinetic (PK) analysis, no additional dose adjustments beyond body weight are required for this age group. The recommended body-weight based dosing regimen in pediatric patients is the same as that in adults.
8.5 Geriatric Use
Clinical studies of ADZYNMA did not include patients 65 years of age and older to determine whether they respond differently from younger patients. Based on the results from population pharmacokinetics analysis, no dose adjustment is required for elderly patients [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
ADZYNMA is a purified bivariant human recombinant “A disintegrin and metalloproteinase with thrombospondin motifs 13” (rADAMTS13) expressed in Chinese Hamster Ovary (CHO) cells using recombinant DNA technology (a mixture of Native rADAMTS13 Q23 and Variant rADAMTS13 R23 with a controlled range of the two variants ratio). ADZYNMA is produced and formulated without the addition of any exogenous raw materials of human or animal origin in the cell culture, purification, or formulation of the final product. The purification process for rADAMTS13 does not include use of a monoclonal antibody reagent. To enhance viral safety, the production process also incorporates two dedicated viral clearance steps – a solvent/detergent treatment step for inactivation and a 20 nm filtration step for removal of viruses. Recombinant ADAMTS13 has a molecular weight of approximately 172 kDa. Proteins that may be present in the final product, other than rADAMTS13, are trace quantities of host cell (CHO) proteins.
ADZYNMA (rADAMTS13) is a sterile, nonpyrogenic, preservative free, white powder supplied in single-dose vials for IV use after reconstitution. Each single-dose vial contains nominally 500 IU or 1500 IU of rADAMTS13, sodium chloride (9.4 mg), calcium chloride dihydrate (1.6 mg), L-histidine (16.7 mg), mannitol (161.4 mg), sucrose (53.8 mg), and polysorbate 80 (2.7 mg). Each vial of ADZYNMA is labeled with the specific number of units of ADAMTS13 potency expressed in IU as measured with a fluorescence resonance energy transfer (FRET) assay using a synthetic 73-amino-acid peptide (FRETS-VWF73). The potency assignment employs an ADAMTS13 concentrate standard that is referenced to a WHO (World Health Organization) international standard for ADAMTS13 concentrates and is evaluated by appropriate methodology to ensure accuracy of the results.
After reconstitution with 5 mL of Sterile Water for Injection, USP, the 500 IU and the 1500 IU vials result in a nominal potency of 100 IU/mL and 300 IU/mL, respectively. All dosage strengths yield a clear, colorless solution, free from particles with a pH of approximately 7.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ADZYNMA is a recombinant form of the endogenous ADAMTS13. ADAMTS13 is a plasma zinc metalloprotease that regulates the activity of von Willebrand factor (VWF) by cleaving large and ultra-large VWF multimers to smaller units and thereby reducing the platelet binding properties of VWF and its propensity to form microthrombi.2,3
12.2 Pharmacodynamics
VWF antigen (VWF:AG) and VWF:ristocetin cofactor activity (VWF:RCo) were used to assess VWF platelet binding activity. Following IV doses of ADZYNMA at the recommended dose, both VWF:AG and VWF:RCo transiently decreased for 1 to 2 days with a 15% to 25% change from baseline.
12.3 Pharmacokinetics
The PK profile of ADZYNMA was determined based on clinical trial ADAMTS13 activity data analyses.
Following single-dose IV administration of ADZYNMA at 5 IU/kg, 20 IU/kg, and 40 IU/kg to adults and adolescents, dose-related increases in individual ADAMTS13 activity were observed and reached a maximum at approximately 1-hour post-infusion or earlier. At a dose of 40 IU/kg the mean (SD) half-life and mean residence time (MRT) in adults and adolescents were 47.8 (13.7) hours and 63.8 (16.0) hours, respectively.
The PK parameters of ADAMTS13 activity following IV administration of ADZYNMA at 40 IU/kg in adults and adolescents are described in Table 3.
Table 3: Pharmacokinetic Parameters of ADAMTS13 Activity following IV Administration of ADZYNMA in cTTP Patients ≥12 Years Old Parameter
(unit)Mean (SD)
Min; Max
(N=23)AUC = area under ADAMTS13 activity-time curve; Cave (0-168 h) = average ADAMTS13 activity from 0 to 168 hours dosing interval; Cmax = maximum ADAMTS13 activity; IR = incremental recovery; MRT = mean residence time; t1/2 = half-life.
Note: 1 IU/mL ADAMTS13 activity corresponds to 100% average normal activity.- *
- N=22
Cmax
(IU/mL)1.15 (0.25)
0.78; 1.56IR
[(IU/mL)/(IU/kg)]0.03 (0.01)
0.02; 0.04t½
(h)47.8 (13.70)
30.1; 90.8AUC0-inf
(IUxh/mL)57.6 (13.9)
37.0; 88.2AUC(0-168 h)
(IUxh/mL)57.2 (13.67)
36.2; 87.2MRT0-inf*
(h)63.8 (16.0)
44.8; 113Cave (0-168 h)
(IU/mL)0.30 (0.07)
0.20; 0.46Duration ADAMTS13 Activity above 10%
(days)5.8 (1.2)
4.5; 8.9ADZYNMA IV administration at 40 IU/kg resulted in approximately 4- to 5-fold higher ADAMTS13 activity exposures (Cmax, AUC) and lower inter-subject variability when compared to plasma-based therapies. Mean time duration above 10% ADAMTS13 activity and mean average ADAMTS13 activity (Cave) were both approximately 3- to 4-fold higher following ADZYNMA IV administration than plasma-based therapies.
Based on Population PK analysis leveraging available data (N=65) from adults, adolescents and pediatric patients below 12 years of age, the mean (SD) steady state Cmax, AUCall, Cave, and duration of ADAMTS13 activity above 10% following IV administration of ADZYNMA every other week in cTTP patients below 12 years of age were 1.02 (0.19) IU/mL, 54.0 (12.2) IU*h/mL, 0.16 (0.04) IU/mL, and 6.8 (2.1) days, respectively.
ADAMTS13 antigen and activity PK characteristics (MRT, Vss, and CL) were similar across age groups in patients with cTTP. Body-weight based ADZYNMA dosing provides similar ADAMTS13 activity PK parameters (Cmax and Cave) across the different age groups including pediatric patients <12 years of age.
Specific Populations
Besides body-weight dosing regimen, no dose adjustment is required since no intrinsic factors such as age, gender, race, baseline estimated glomerular filtration rate (eGFR), and baseline bilirubin were identified as covariates impacting ADAMTS13 PK.
Pediatric Patients
Population pharmacokinetics (PK) analysis leveraging available data from adults, adolescents and pediatric patients below 12 years of age (N=65), suggests comparable ADAMTS13 activity exposures between patients below 12 years of age and patients aged 12 years and above receiving the recommended ADZYNMA dosing regimen [see Use in Specific Populations (8.4)].
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of ADZYNMA.
Thirteen (one patient in Study 1 and twelve patients in a long-term extension study) of 67 patients treated prophylactically with ADZYNMA with confirmed cTTP tested positive for low-titer binding antibodies against ADAMTS13 with no observable clinical impact on the safety or efficacy of ADZYNMA, and no increase in antibody titers over time. No cTTP patients tested positive for neutralizing antibodies against ADAMTS13.
Because of the low occurrence of ADA, the effect of these antibodies on the PK, PD, safety, and/or efficacy of rADAMTS13 products is unknown [see Warnings and Precautions (5.2)].
There are no data on immunogenicity in previously untreated patients (subjects naïve to plasma-based products), therefore, the risk of immunogenicity for naïve subjects to this drug product is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or mutagenicity studies have been conducted with ADZYNMA to assess its mutagenic or carcinogenic potential.
No adverse effects on fertility were observed in female rats administered 80, 200, or 400 IU/kg ADZYNMA every third day for 2 weeks before mating through Day 16.
-
14 CLINICAL STUDIES
ADZYNMA was studied in a global, prospective, randomized, active-controlled, open-label, multicenter, two-period crossover study followed by a single arm continuation period (Study 1) evaluating the efficacy and safety of the prophylactic and on demand ERT with ADZYNMA compared to plasma-based therapies in patients with cTTP.
Prophylactic Enzyme Replacement Therapy in patients with cTTP
The efficacy of ADZYNMA in the prophylactic treatment of patients with cTTP was evaluated in Study 1, in 46 patients who were randomized to receive 6 months of treatment with either 40 IU/kg of ADZYNMA or plasma-based therapies (Period 1), then crossed over to the other treatment for 6 months (Period 2). Thirty-five patients have entered the 6-month single arm period with ADZYNMA (Period 3).
The median (min-max) age of patients was 32.5 years (range 3-58 years), with a mean weight of 67.6 kg. Most patients were white (65.2%), not Hispanic or Latino (80.4%) and were female (58.7%). Twenty of the 27 female patients (74.1%) were of child-bearing potential. Baseline characteristics are listed in Table 4.
Table 4: Baseline Characteristics of the Prophylactic Cohort Characteristic Prophylactic Cohort*
(N=46)- *
- The prophylactic cohort includes the patients who were originally enrolled in the prophylaxis cohort and the patients who moved to the prophylaxis cohort from the on demand cohort.
- †
- Acute TTP events were defined in protocol by a drop in platelet count (≥50% of baseline or a platelet count <100,000/μL) and an elevation of lactate dehydrogenase (LDH) (>2 ×baseline or >2 ×upper limit normal (ULN)).
cTTP Pre-Study Treatment 45 (97.8) Fresh Frozen Plasma (FFP) [n (%)] 32 (69.6) Solvent/Detergent Treated Plasma [n (%)] 10 (21.7) FVIII-VWF Concentrations [n (%)] 3 (6.5) Acute TTP Events (in the 12 Months prior to screening) [n (%)]† Yes 8 (17.4) No 38 (82.6) The efficacy of prophylactic treatment with ADZYNMA in patients with cTTP was demonstrated based on the incidence of protocol defined acute and subacute TTP events and TTP manifestations (see Table 5), as well as the incidence of supplemental doses prompted by subacute TTP events over a 6-month time period.
No patients receiving ADZYNMA had an acute TTP event throughout the study, including Period 3 (with a median duration of exposure to ADZYNMA of 14 months for patients 12 to <18 years of age and patients ≥18 years of age; and 4 and 1 months in patients 6 to <12 and <6 years of age, respectively). One acute TTP event occurred in a patient receiving plasma-based therapies (FFP) prophylactically during Period 1 (see Table 5).
No subacute TTP events were reported in patients receiving ADZYNMA during Periods 1 and 2. In Period 3, two patients receiving ADZYNMA prophylaxis had two subacute events of which one was treated with four supplemental doses, 2 of FFP and 2 of ADZYNMA. Four patients receiving plasma-based therapies had five subacute TTP events in Periods 1 and 2. A total of seven supplemental doses, 2 of FVIII-VWF concentrate, 1 of FFP and 4 of ADZYNMA were given to three of these patients (see Table 5).
Table 5: Prophylactic Cohort Efficacy Results in cTTP Patients ≥12 Years of Age (Periods 1 and 2) ADZYNMA
N=37Plasma-Based Therapies
N=38SD = standard deviation; TTP = thrombotic thrombocytopenic purpura. - *
- Acute TTP events were defined by a drop in platelet count (≥50% of baseline or a platelet count <100,000/μL) and an elevation of lactate dehydrogenase (LDH) (>2× baseline or >2×upper limit normal (ULN)).
- †
- Annualized event rate for a subject = number of events/duration of the observation period (years). Data shown are non-model based.
- ‡
- Subacute events were defined by a thrombocytopenia event or a microangiopathic hemolytic anemia event; and organ-specific signs and symptoms including but not limited to renal dysfunction events, neurological symptoms events, fever, fatigue/lethargy, and/or abdominal pain.
- §
- TTP manifestations not shown also included renal dysfunction, neurological symptoms and abdominal pain. The clinical significance of the observed difference for the presented manifestations is unknown.
- ¶
- Thrombocytopenia events were defined as a drop in platelet count ≥25% of baseline or a platelet count <150,000/μL.
- #
- Microangiopathic hemolytic anemia events were defined as an elevation of LDH >1.5 ×baseline or >1.5 xULN.
Acute TTP events* Number of subjects with event
(number of events)0
(0)1
(1)Mean annualized event rate (SD)† 0 (0.000) 0.05 (0.304) Subacute TTP events‡ Number of subjects with event
(number of events)0
(0)4
(5)Mean annualized event rate (SD)† 0 (0.000) 0.27 (0.836) TTP manifestations§ Thrombocytopenia events¶ Number of subjects with event
(number of events)9
(30)19
(75)Mean annualized event rate (SD)† 2.0 (4.706) 4.44 (6.312) Microangiopathic hemolytic anemia events# Number of subjects event
(number of events)5
(7)11
(20)Mean annualized event rate (SD)† 0.38 (1.028) 1.47 (3.274) Overall, the efficacy results for ADZYNMA were consistent throughout the study, including Period 3 and across age groups. For outcome of ADZYNMA use in pregnancy [see Use in Specific Populations (8.1)].
On-Demand Enzyme Replacement Therapy
The efficacy of the on-demand (OD) enzyme replacement therapy was evaluated based on the proportion of acute TTP events responding to ADZYNMA in both the Prophylactic and the OD cohorts throughout the duration of the study.
An acute TTP event responding to ADZYNMA was defined as a resolved TTP event when platelet count was ≥150,000/μL or platelet count was within 25% of baseline, whichever occurs first, and LDH ≤1.5 x baseline or ≤1.5 x ULN, without requiring the use of another ADAMTS13-containing agent.
Five adult patients (≥18 years of age) enrolled in the OD cohort and had a total of six acute TTP events. Of these five patients, two patients were randomized to receive on-demand treatment with ADZYNMA and three patients were randomized to receive plasma-based therapies. All 6 acute TTP events resolved after treatment with either ADZYNMA or plasma-based therapies.
-
15 REFERENCES
- Asmis, L. M., Serra, A., Krafft, A., Licht, A., Leisinger, E., Henschkowski-Serra, J., et al. 2022. Recombinant ADAMTS13 for Hereditary Thrombotic Thrombocytopenic Purpura. N Engl J Med, 387, 2356-2361.
- Alwan, F., C. Vendramin, R. Liesner, A. Clark, W. Lester, T. Dutt, W. Thomas, R. Gooding, T. Biss, H. G. Watson, N. Cooper, R. Rayment, T. Cranfield, J. J. van Veen, Q. A. Hill, S. Davis, J. Motwani, N. Bhatnagar, N. Priddee, M. David, M. P. Crowley, J. Alamelu, H. Lyall, J. P. Westwood, M. Thomas and M. Scully (2019). “Characterization and treatment of congenital thrombotic thrombocytopenic purpura.” Blood 133(15): 1644-1651.
- Sukumar, S., B. Lämmle and S. R. Cataland (2021). “Thrombotic Thrombocytopenic Purpura: Pathophysiology, Diagnosis, and Management.” J Clin Med 10(3): 536.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ADZYNMA (rADAMTS13) is a sterile, nonpyrogenic, preservative free, white powder supplied in single-dose vials packaged with:
- one vial with 5 mL sterile water for injection, USP
- one BAXJECT II Hi-FLOW needleless transfer device
- one syringe
- one 25 gauge infusion set
- two individually packaged alcohol swabs
- one package insert
ADZYNMA (rADAMTS13) is available in the following strengths:
Color Code Nominal rADAMTS13 Potency rADAMTS13 vial NDC Carton NDC Syringe Fill Size Eggplant 500 IU 64764-130-01 64764-140-05 10 mL Marigold 1500 IU 64764-135-01 64764-145-05 20 mL The actual ADAMTS13 potency in international units is printed on the label of each ADZYNMA vial and carton.
Components are not made with natural rubber latex.
Storage and Handling
- Store at refrigerated temperature 2°C to 8°C (36°F to 46°F) for up to 36 months from the date of manufacture until expiration date stated on the ADZYNMA vial label and carton. Within this period, ADZYNMA may be stored at room temperature not to exceed 30°C/86°F for a period up to 6 months. After storage at room temperature, do not return to the refrigerator.
- Do not use beyond the expiration date printed on the ADZYNMA vial label or carton or if not stored properly.
- Do not freeze.
- Store in the original box and protect from extreme exposure to light.
- Use reconstituted product immediately or within 3 hours after reconstitution when stored at room temperature.
- Do not use if the solution in the syringe is cloudy or contains particles.
- Discard any unused reconstituted product after 3 hours.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient:
- To read the FDA-approved patient labeling (Patient Information).
- About early signs of hypersensitivity reactions, including tachycardia, tightness of the chest, wheezing and/or acute respiratory distress, hypotension, generalized urticaria, pruritus, rhinoconjunctivitis, angioedema, lethargy, nausea, vomiting, paresthesia, and restlessness. Advise patients to discontinue use of the product if these symptoms occur and seek immediate emergency treatment with appropriate supportive care.
- To consult with their physicians or healthcare provider prior to traveling. While traveling, advise patients to bring an adequate supply of ADZYNMA based on their current regimen of treatment.
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Takeda Pharmaceuticals U.S.A., Inc.
Cambridge, MA 02142
U.S. License No. 1898ADZYNMA and
 are trademarks of Takeda Pharmaceuticals International AG.
are trademarks of Takeda Pharmaceuticals International AG. BAXJECT® is a registered trademark of Baxalta Incorporated.
TAKEDA® and
 are registered trademarks of Takeda Pharmaceutical Company Limited.
are registered trademarks of Takeda Pharmaceutical Company Limited.Patented: see https://www.takeda.com/en-us/patents
©2024 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
ADZ379 R2
-
PATIENT PACKAGE INSERT
Patient Information
ADZYNMA (ad-zin-muh)
(ADAMTS13, recombinant-krhn)
Lyophilized Powder for Injection, for Intravenous Use
Single-Dose VialThis Patient Information has been approved by the U.S. Food and Drug Administration. Revised 6/2024 Read this Patient Information before you start using ADZYNMA. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. What is ADZYNMA?
ADZYNMA is a prescription medicine used to prevent or treat blood clots in patients with congenital thrombotic thrombocytopenic purpura (cTTP). This medicine replaces low amounts of the enzyme (ADAMTS13) in the body.Who should not take ADZYNMA?
Do not take ADZYNMA if you have had a life-threatening allergic reaction to ADZYNMA, or any of the ingredients in ADZYNMA. See the end of this Patient Information for a complete list of ingredients in ADZYNMA.Before you take ADZYNMA, tell your healthcare provider about all of your medical conditions, including if you: - have any allergies, including allergies to hamsters.
- are pregnant or plan to become pregnant. It is not known if ADZYNMA will harm your unborn baby. Tell your healthcare provider right away if you become pregnant while receiving ADZYNMA.
- are breastfeeding or plan to breastfeed. It is not known if ADZYNMA passes into breast milk. Talk to your healthcare provider about the best way to feed your baby if you take ADZYNMA.
How is ADZYNMA given?
ADZYNMA will be slowly injected into your vein (intravenous injection).What are the possible side effects of ADZYNMA?
You can have an allergic reaction to ADZYNMA.
Call your healthcare provider right away and stop treatment if you get a rash or hives, itching, tightness of the throat, chest pain or tightness, difficulty breathing, lightheadedness, dizziness, nausea, or fainting.
The most common side effects that have been reported with ADZYNMA include: headache, diarrhea, migraine, abdominal pain, nausea, upper respiratory tract infection, dizziness, and vomiting.
Tell your healthcare provider about any side effect that bothers you or does not go away.
These are not all of the possible side effects of ADZYNMA. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.What else should I know about ADZYNMA and hereditary ADAMTS13 deficiency (cTTP)?
Your body can form inhibitors to ADAMTS13. An inhibitor is part of the body’s normal defense system. If you form inhibitors, they may stop ADZYNMA from working properly. Consult with your healthcare provider to make sure you are carefully monitored with blood tests for the development of inhibitors to ADAMTS13.General information about the safe and effective use of ADZYNMA
Medicines are sometimes prescribed for purposes other than those listed here. Do not use ADZYNMA for a condition for which it is not prescribed. Do not give ADZYNMA to other people, even if they have the same symptoms you have. It may harm them.What are the ingredients in ADZYNMA?
Active ingredient: recombinant ADAMTS13.
Inactive ingredients: sodium chloride, calcium chloride dihydrate, L-histidine, mannitol, sucrose, and polysorbate 80.Resources at Takeda available to the patients:
For more product information on ADZYNMA, please visit www.ADZYNMA.com or call 1-877-TAKEDA-7 (1-877-825-3327).
For information on additional Takeda patient resources, please visit www.ADZYNMA.com.
Manufactured by:
Takeda Pharmaceuticals U.S.A., Inc.
Cambridge, MA 02142
U.S. License No. 1898ADZYNMA and
 are trademarks of Takeda Pharmaceuticals International AG.
are trademarks of Takeda Pharmaceuticals International AG.BAXJECT® is a registered trademark of Baxalta Incorporated. TAKEDA® and
 are registered trademarks of Takeda Pharmaceutical Company Limited.
are registered trademarks of Takeda Pharmaceutical Company Limited.Patented: see https://www.takeda.com/en-us/patents ©2024 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved. ADZ379 R2 -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
ADZYNMA
(ad-zin-muh)
(ADAMTS13, recombinant-krhn)
Lyophilized Powder for Injection, for Intravenous Use
Single-Dose Vial
The following information is intended for medical or healthcare professionals only.This Instructions for Use contains information on how to reconstitute and infuse ADZYNMA. This Instructions for Use is intended for the Healthcare Provider. Treatment with ADZYNMA should be prescribed and administered by a healthcare professional experienced in the treatment of blood disorders.
Important:
- For intravenous injection after reconstitution only.
- Use aseptic technique (clean and germ-free) throughout the procedure.
- Check the expiration date of the product prior to use.
- Do not use ADZYNMA if the expiration date has passed.
Storage of ADZYNMA
- Store ADZYNMA at refrigeration temperature of 36°F to 46°F [2°C to 8°C] until the expiration date stated on the carton.
- Can be stored at room temperature not to exceed 86°F/30°C for a period of 6 months, do not return to the refrigerator.
- Do not use beyond the expiration date printed on the carton or vial or if not stored properly.
- Do not freeze.
- Store vials in the original package to protect from light.
Reconstitution of ADZYNMA Using the BAXJECT II Hi-Flow Needleless Transfer Device
- 1.
- Prepare a clean, germ-free, flat surface and gather all the materials you will need for the reconstitution and infusion. Figure A depicts the materials provided in the carton box.
Figure A
- 2.
-
Do not use ADZYNMA if the expiration date has passed.
- Use ADZYNMA within 3 hours after reconstitution and keep at room temperature not to exceed 86°F/30°C. Do not store at any other temperature. Discard any unused reconstituted product if not used within 3 hours after reconstitution.
- 3.
- Allow the vials of ADZYNMA and diluent to reach room temperature before use.
- If the patient needs more than one vial of ADZYNMA per injection, reconstitute each vial according to the instructions stated under “Reconstitution of ADZYNMA Using the BAXJECT II Hi-Flow Needleless Transfer Device”.
- Inspect the reconstituted ADZYNMA solution for particulate matter and discoloration prior to administration. The solution should be clear and colorless in appearance.
- Do not administer if particulate matter or discoloration is observed.
- 4.
- Wash and dry your hands thoroughly, and put on clean exam gloves.
- 5.
- Remove plastic caps from the ADZYNMA and diluent vials and place the vials on a flat surface (Figure B).
Figure B
- 6.
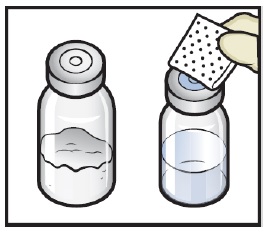
- Wipe the rubber stoppers with an alcohol swab and allow them to dry prior to use. (Figure C).
Figure C
- 7.
- Open the BAXJECT II Hi-Flow device package by peeling away the lid, without touching the inside (Figure D).
- 8.
- Turn the package with the BAXJECT II Hi-Flow device upside down and place it over the top of the diluent vial. Press straight down until the clear plastic spike pierces through the diluent vial stopper (Figure E).
Figure E
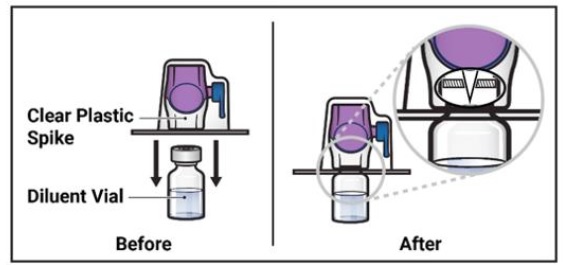
- 9.
- Grip the BAXJECT II Hi-Flow device package at its edge and pull the package off the device (Figure F).
- 10.
- Turn the system over so that the diluent vial is now on top. Press the BAXJECT II Hi-Flow device straight down until the purple plastic spike pierces through the ADZYNMA powder vial stopper (Figure G). The vacuum will draw the diluent into the ADZYNMA powder vial.
- 11.
- Swirl the connected vials gently and continuously until the powder is completely dissolved (Figure H).
- 12.
- Visually inspect the reconstituted solution for particulate matter before administration. The solution should be clear and colorless in appearance.
- Do not use the product if particulate matter or discoloration is observed.
- 13.
- If the dose requires more than one vial of ADZYNMA, repeat step 1 to step 12 to reconstitute each vial.
- Use a different BAXJECT II Hi-Flow device to reconstitute each vial of ADZYNMA and diluent.
Administration of ADZYNMA
- 14.
- Take off the blue cap from the BAXJECT II Hi-Flow device (Figure I). Attach a Luer-lock syringe (Figure J).
- 15.
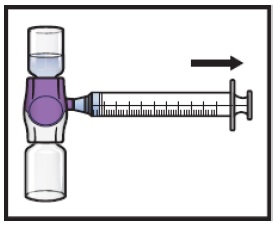
-
Turn the system upside down (ADZYNMA vial is now on top). Draw the reconstituted solution into the syringe by pulling the plunger back slowly (Figure K).
- Use ADZYNMA within 3 hours after reconstitution and keep at room temperature (not to exceed 86°F/30°C). Do not store at any other temperature. Discard any unused reconstituted product if not used within 3 hours after reconstitution.
-
Do not administer ADZYNMA in the same tubing or container at the same time with other medicinal products for infusion.
Figure K
- 16.
- If a patient is to receive more than one vial of ADZYNMA, the contents of multiple vials can be drawn into the same syringe. Repeat this process for all reconstituted vials of ADZYNMA until the total volume to be administered is reached.
- 17.
- Disconnect the syringe and attach a suitable injection needle or an infusion set.
- 18.
- Point the needle up and remove any air bubbles by gently tapping the syringe with your finger and slowly and carefully pushing air out of the syringe and needle.
- 19.
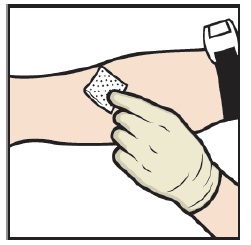
- Apply a tourniquet and clean the chosen infusion site with an alcohol swab (Figure L).
Figure L
- 20.
- Insert the needle into the vein and remove the tourniquet.
- 21.
- Infuse the reconstituted ADZYNMA slowly, at a rate of 2 to 4 mL per minute (Figure M).
- 22.
- Take the needle out of the vein, place a cotton ball or gauze on the infusion site, and apply pressure for several minutes to stop bleeding.
- Do not recap the needle.
- 23.
- Place the needle, syringe, and empty vials in a puncture-resistant sharps container.
- Do not dispose of syringes and needles in the regular waste.
Disposing of ADZYNMA
- Vials are for single use only. Any remaining solution in the vial must not be used and must be discarded in accordance with local requirements. Dispose used vials and other supplies in an FDA cleared sharps disposal container.
- Do not dispose of syringes and needles in the regular waste.
ADZYNMA and
 are trademarks of Takeda Pharmaceuticals International AG.
are trademarks of Takeda Pharmaceuticals International AG.
BAXJECT® is a registered trademark of Baxalta Incorporated.
TAKEDA® and the are registered trademarks of Takeda Pharmaceutical Company Limited.
are registered trademarks of Takeda Pharmaceutical Company Limited.Patented: see https://www.takeda.com/en-us/patents
Manufactured by:
Takeda Pharmaceuticals U.S.A., Inc.
Cambridge, MA 02142
U.S. License No. 1898This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 6/2024 -
PRINCIPAL DISPLAY PANEL - 500 IU Kit Carton
NDC 64764-140-05
Rx Only
ADZYNMA
ADAMTS13, recombinant-krhnFor Intravenous Use After Reconstitution Only
500 IU
NominalSingle-dose vial
Dosage: See prescribing information. Prior to administration of ADZYNMA,
read the instructions for use.Storage: Store refrigerated at 36°F to 46°F (2°C to 8°C) until the expiration date
stated on the carton, do not freeze. Can be stored at room temperature not to
exceed 86°F/30°C for a period of 6 months, do not return to refrigerator.
Store in the original carton to protect from light. See prescribing information.Reconstitution: Use 5 mL Sterile Water for Injection, USP. Gently swirl, do not shake.
Do not refrigerate after reconstitution. Use within 3 hours of reconstitution.
Discard unused portion.Keep out of the reach of children.
Date removed from
refrigerator and placed
at room temperature
Date _________________
-
PRINCIPAL DISPLAY PANEL - 1500 IU Kit Carton
NDC 64764-145-05
Rx Only
ADZYNMA
ADAMTS13, recombinant-krhnFor Intravenous Use After Reconstitution Only
1500 IU
NominalSingle-dose vial
Dosage: See prescribing information. Prior to administration of ADZYNMA,
read the instructions for use.Storage: Store refrigerated at 36°F to 46°F (2°C to 8°C) until the expiration date
stated on the carton, do not freeze. Can be stored at room temperature not to
exceed 86°F/30°C for a period of 6 months, do not return to refrigerator.
Store in the original carton to protect from light. See prescribing information.Reconstitution: Use 5 mL Sterile Water for Injection, USP. Gently swirl, do not shake.
Do not refrigerate after reconstitution. Use within 3 hours of reconstitution.
Discard unused portion.Keep out of the reach of children.
Date removed from
refrigerator and placed
at room temperature
Date _________________
-
INGREDIENTS AND APPEARANCE
ADZYNMA
apadamtase alfa kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64764-140 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-140-05 1 in 1 CARTON 11/09/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 500 [iU] Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 ADZYNMA
apadamtase alfa injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:64764-130 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Apadamtase Alfa (UNII: U5SFU33XUX) (Apadamtase Alfa - UNII:U5SFU33XUX) Apadamtase Alfa 500 [iU] in 1 [iU] Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Calcium Chloride (UNII: M4I0D6VV5M) Histidine (UNII: 4QD397987E) Mannitol (UNII: 3OWL53L36A) Sucrose (UNII: C151H8M554) Polysorbate 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-130-01 500 [iU] in 1 VIAL, SINGLE-DOSE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125795 11/09/2023 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC:64764-515 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-515-50 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125795 11/09/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125795 11/09/2023 ADZYNMA
apadamtase alfa kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64764-145 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-145-05 1 in 1 CARTON 11/09/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 1500 [iU] Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 ADZYNMA
apadamtase alfa injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:64764-135 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Apadamtase Alfa (UNII: U5SFU33XUX) (Apadamtase Alfa - UNII:U5SFU33XUX) Apadamtase Alfa 1500 [iU] in 1 [iU] Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Calcium Chloride (UNII: M4I0D6VV5M) Histidine (UNII: 4QD397987E) Mannitol (UNII: 3OWL53L36A) Sucrose (UNII: C151H8M554) Polysorbate 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-135-01 1500 [iU] in 1 VIAL, SINGLE-DOSE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125795 11/09/2023 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC:64764-515 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-515-50 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125795 11/09/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125795 11/09/2023 Labeler - TAKEDA PHARMACEUTICALS AMERICA, INC. (039997266) Establishment Name Address ID/FEI Business Operations Takeda Manufacturing Singapore Pte Ltd 659910011 API MANUFACTURE(64764-130, 64764-140, 64764-135, 64764-145) , ANALYSIS(64764-130, 64764-140, 64764-135, 64764-145) Establishment Name Address ID/FEI Business Operations Takeda Manufacturing Austria AG 300466733 ANALYSIS(64764-130, 64764-140, 64764-135, 64764-145) Establishment Name Address ID/FEI Business Operations Takeda Manufacturing Austria AG 300434670 MANUFACTURE(64764-130, 64764-140, 64764-135, 64764-145) , ANALYSIS(64764-130, 64764-140, 64764-135, 64764-145) , PACK(64764-130, 64764-140, 64764-135, 64764-145, 64764-515) , LABEL(64764-130, 64764-140, 64764-135, 64764-145, 64764-515) Establishment Name Address ID/FEI Business Operations SGS Lab Simon 283063907 ANALYSIS(64764-130, 64764-140, 64764-135, 64764-145) Establishment Name Address ID/FEI Business Operations BioReliance Ltd 505004556 ANALYSIS(64764-130, 64764-140, 64764-135, 64764-145) Establishment Name Address ID/FEI Business Operations Sigma-Aldrich Pte Ltd 894684393 ANALYSIS(64764-130, 64764-140, 64764-135, 64764-145) Establishment Name Address ID/FEI Business Operations Shire Human Genetic Therapies Inc. 831834762 ANALYSIS(64764-130, 64764-140, 64764-135, 64764-145) Establishment Name Address ID/FEI Business Operations Charles River Laboratories Germany GmbH 344516158 ANALYSIS(64764-130, 64764-140, 64764-135, 64764-145) Establishment Name Address ID/FEI Business Operations OFI Technologie & Innovation GmbH 300681124 ANALYSIS(64764-130, 64764-140, 64764-135, 64764-145) Establishment Name Address ID/FEI Business Operations Siegfried Hameln GmbH 315869123 MANUFACTURE(64764-515) , ANALYSIS(64764-515) Establishment Name Address ID/FEI Business Operations Hameln RDS 644165029 ANALYSIS(64764-515)