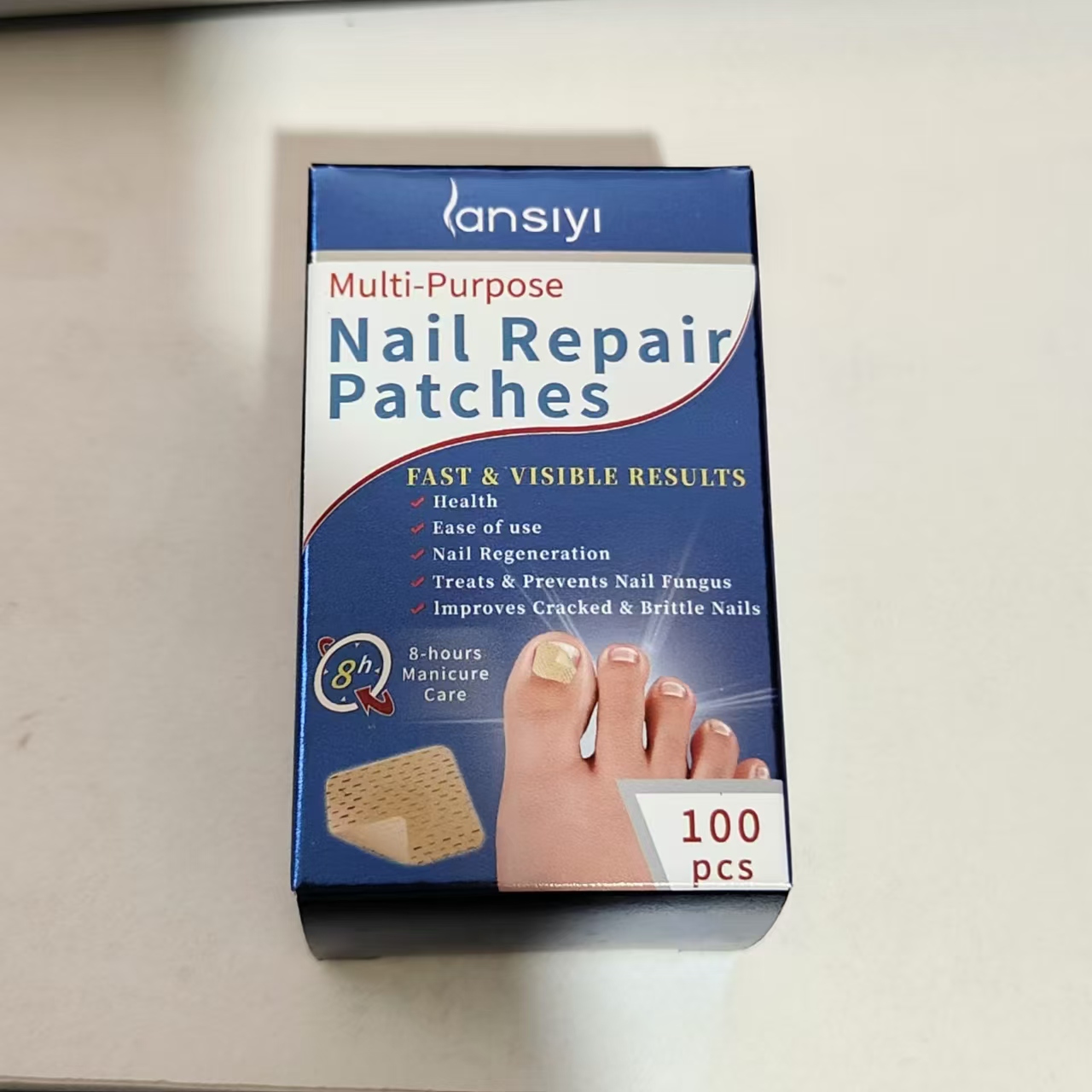
Label: NAIL REPAIR PATCHES- nail stickers ointment
- NDC Code(s): 84727-001-01
- Packager: Guangzhou Baishili Trading Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- BOXED WARNING (What is this?)
- Active ingredients
- Usage
- Uses
- Warning
- Warning
- Warning
- Warning
- Keep out of reach of children.
- DOSAGE & ADMINISTRATION SECTION
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NAIL REPAIR PATCHES
nail stickers ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84727-001 Route of Administration CUTANEOUS, AURICULAR (OTIC), BUCCAL, CONJUNCTIVAL, DENTAL, ELECTRO-OSMOSIS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TEA TREE OIL (UNII: VIF565UC2G) (TEA TREE OIL - UNII:VIF565UC2G) TEA TREE OIL 100 mg in 5 g GARLIC (UNII: V1V998DC17) (GARLIC - UNII:V1V998DC17) GARLIC 1000 mg in 5 g ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 1000 mg in 5 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 100 mg in 5 g Product Characteristics Color yellow Score no score Shape RECTANGLE Size 15mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84727-001-01 100 g in 1 BOX; Type 0: Not a Combination Product 09/16/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 09/16/2024 Labeler - Guangzhou Baishili Trading Co., Ltd (709042161)