Label: ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD PIMIENTA CALIENTE) - BROWN- octinoxate lipstick
ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD VINO CAUTIVANTE) - BROWN- octinoxate lipstick
ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROSA VIVA) - PINK- octinoxate lipstick
ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD CORAL EN .......SIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD BERRY BALCANIC) - RED- octinoxate lipstick
ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROSA OBSESSION) - PINK- octinoxate lipstick
ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROJO EXHUBERANTE) - RED- octinoxate lipstick
ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20- octinoxate kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 13537-729-01, 13537-729-02, 13537-730-03, 13537-730-04, view more13537-731-05, 13537-731-06, 13537-732-07, 13537-732-08, 13537-733-09, 13537-733-10, 13537-734-11, 13537-734-12, 13537-735-13, 13537-735-14, 13537-736-15, 13537-736-16, 13537-737-17, 13537-737-18, 13537-738-19, 13537-738-20, 13537-739-21, 13537-739-22, 13537-740-23, 13537-740-24, 13537-741-23, 13537-741-24, 13537-742-23, 13537-742-24, 13537-743-23, 13537-743-24, 13537-744-23, 13537-744-24, 13537-745-23, 13537-745-24, 13537-746-23, 13537-746-24, 13537-747-27, 13537-747-28 - Packager: Ventura Corporation LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 4, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
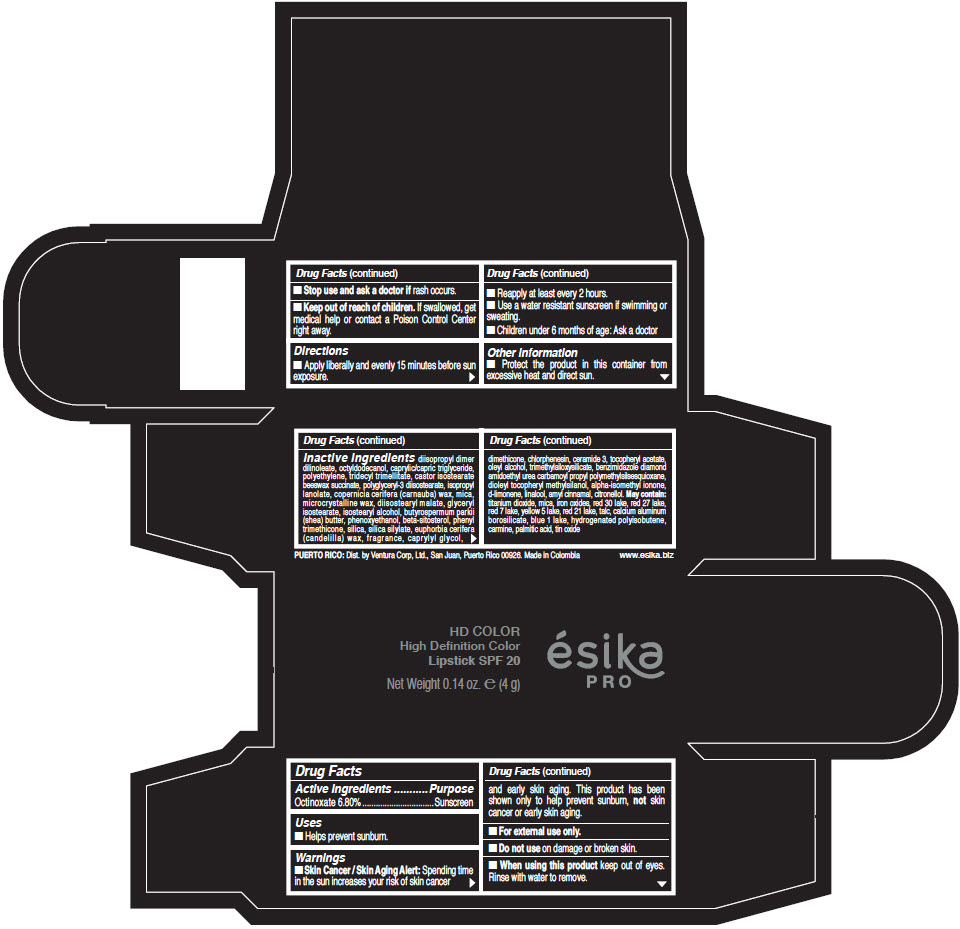
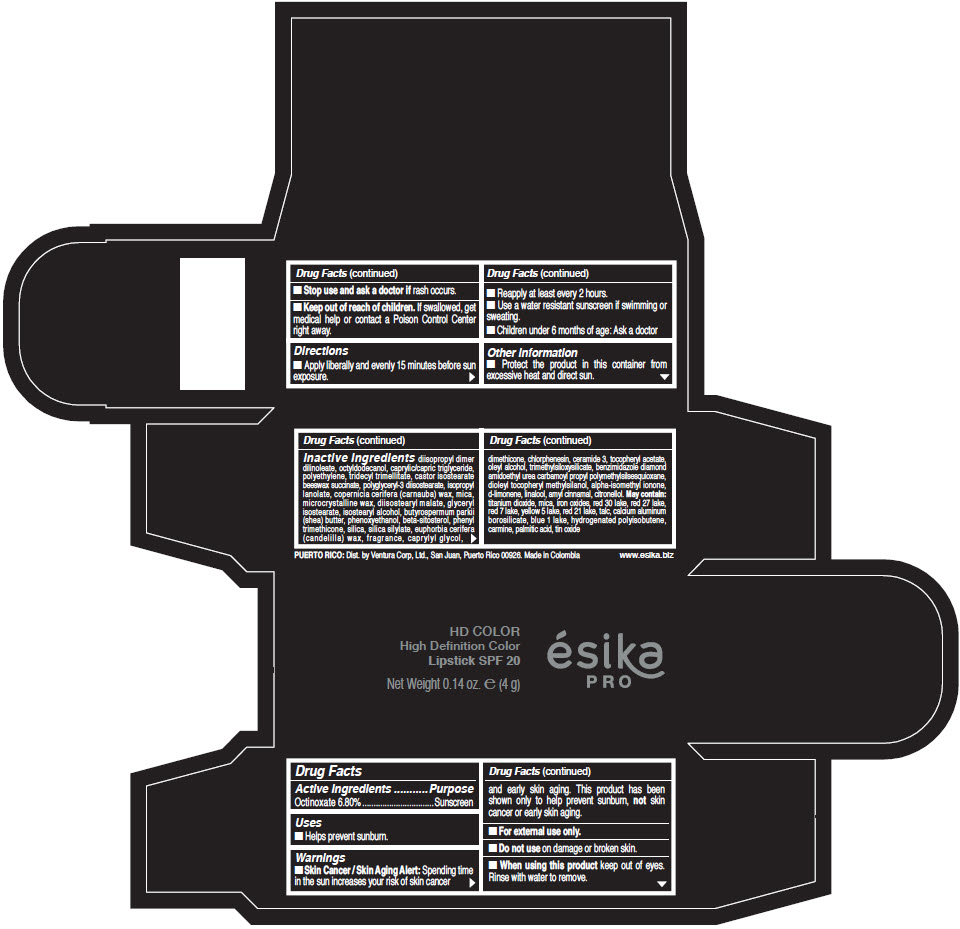
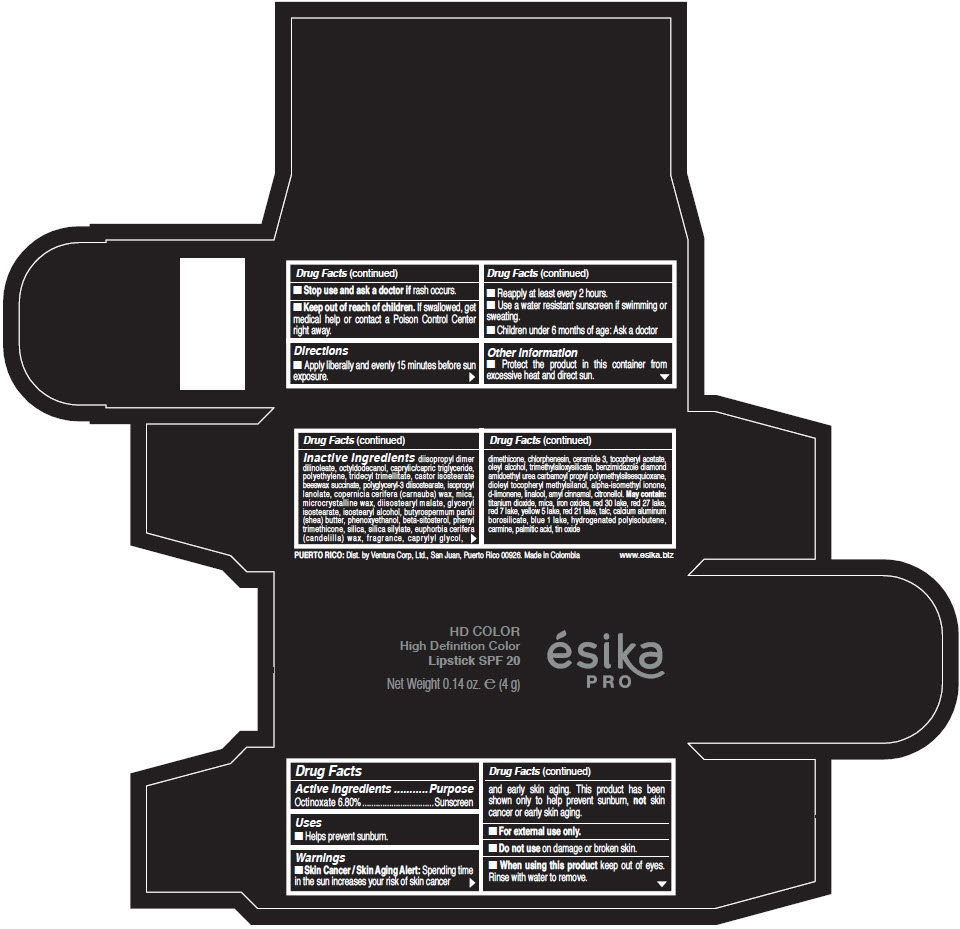
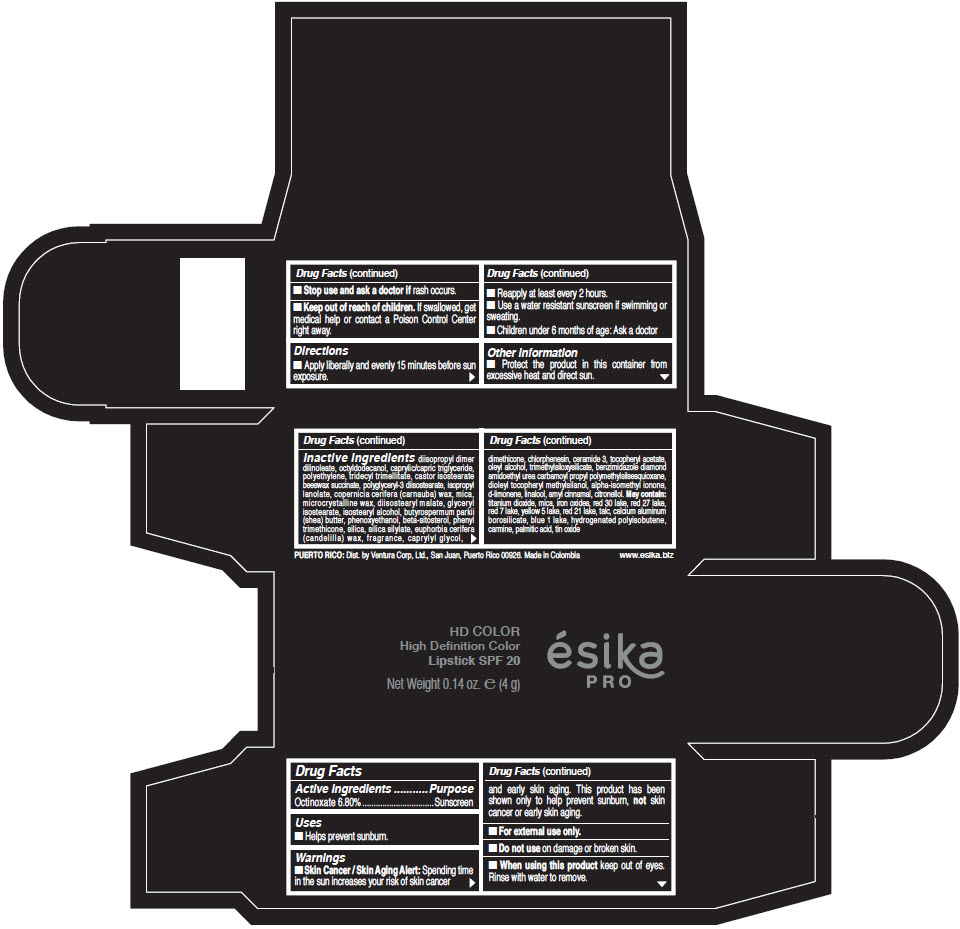
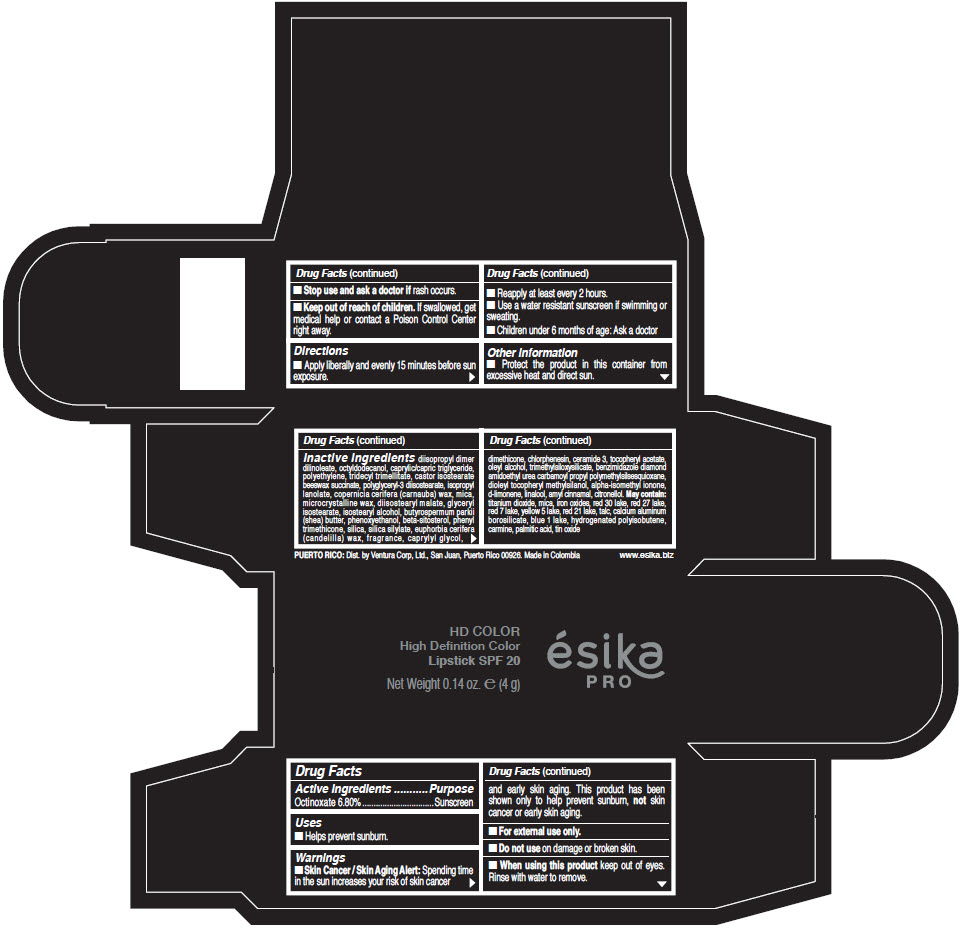
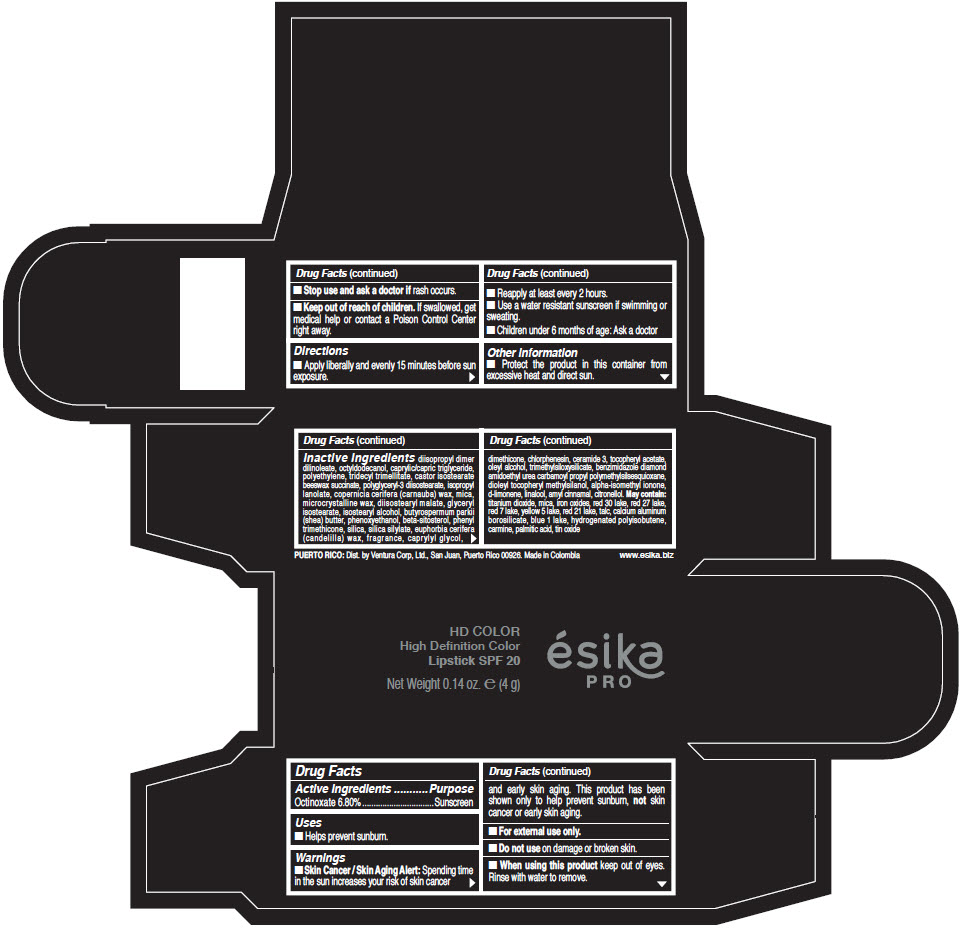
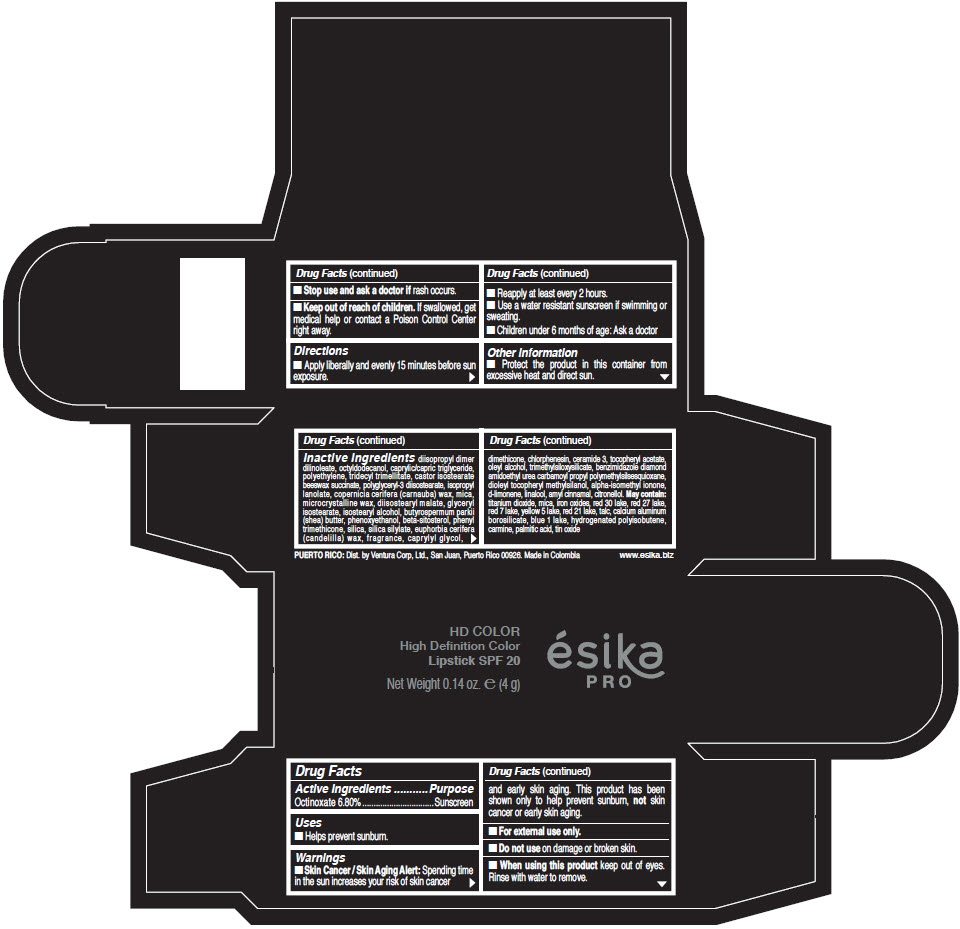
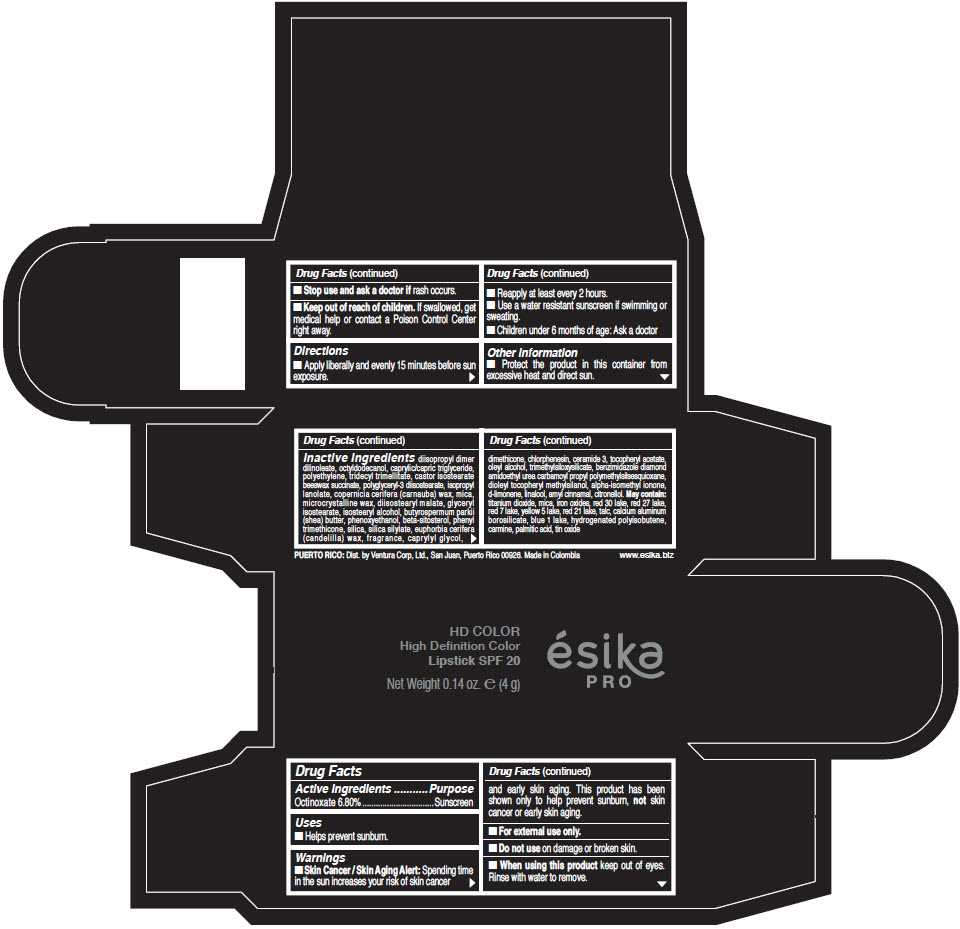
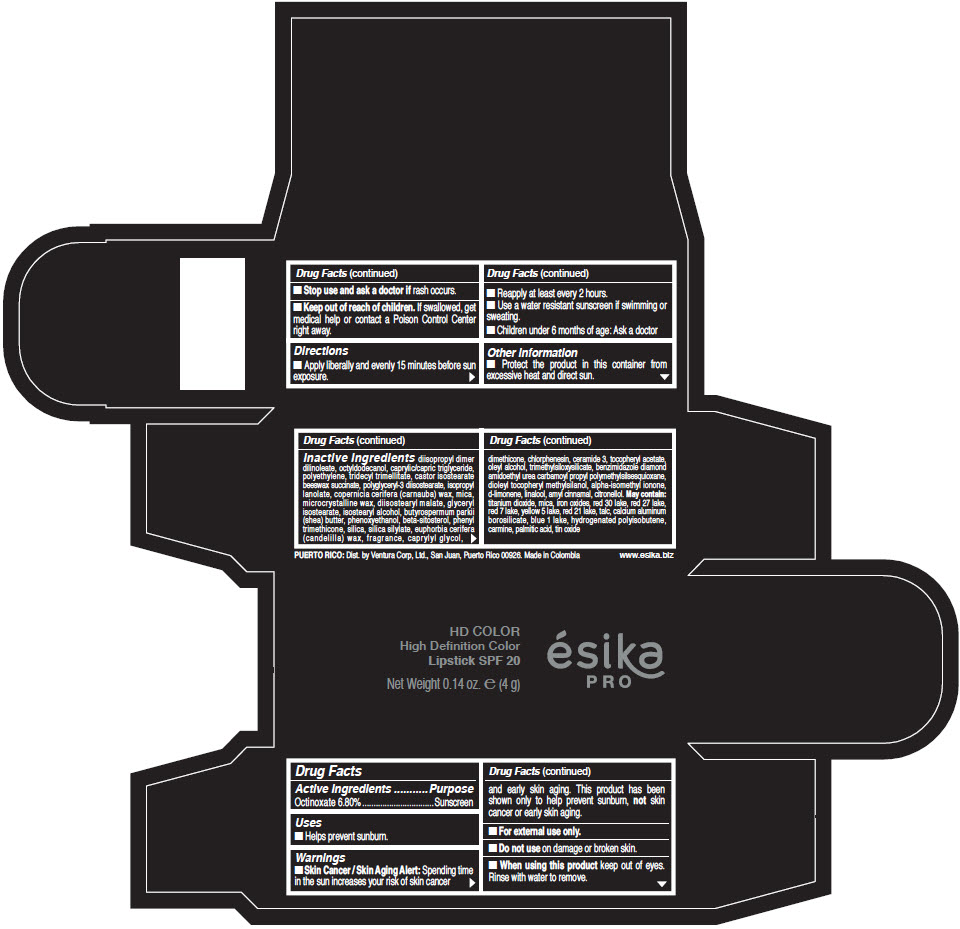
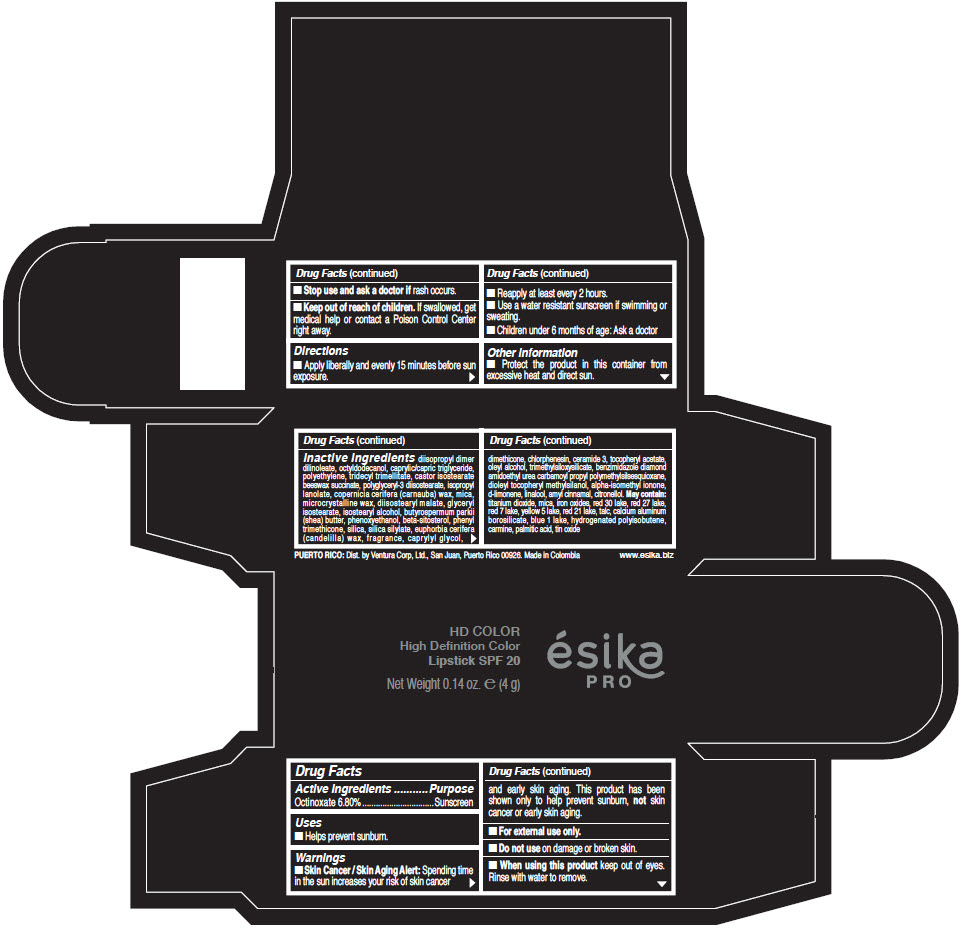
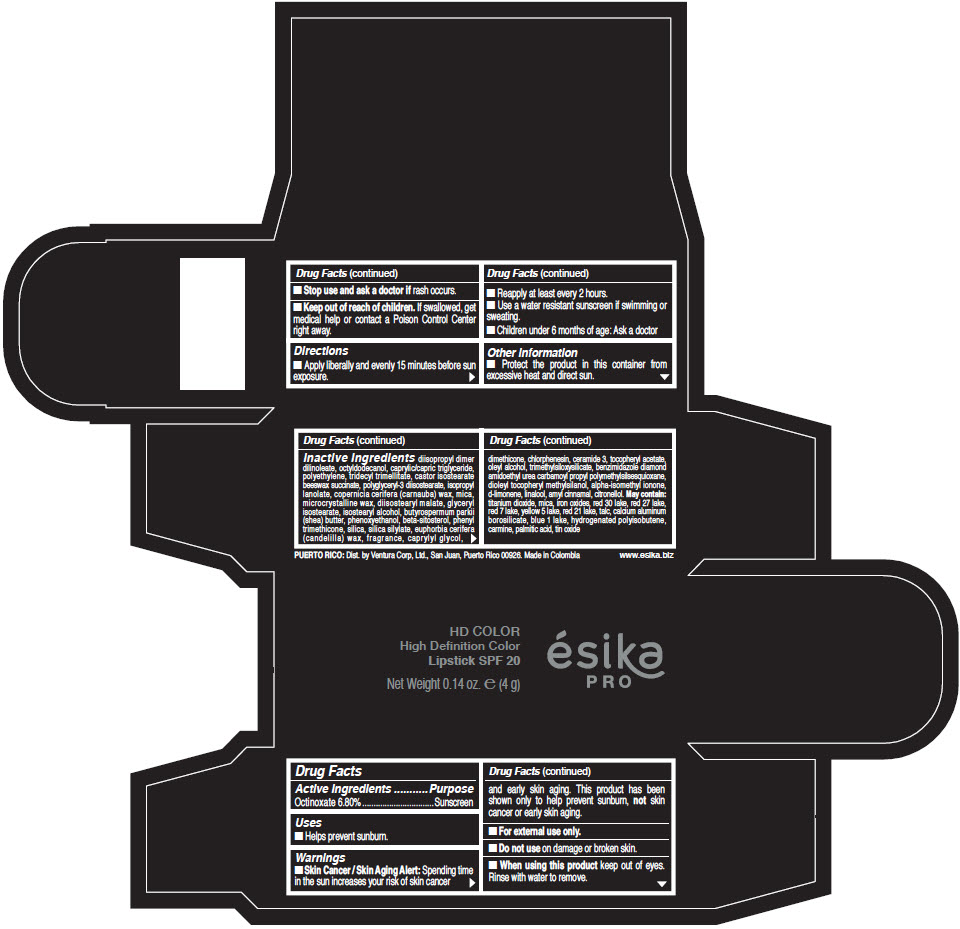
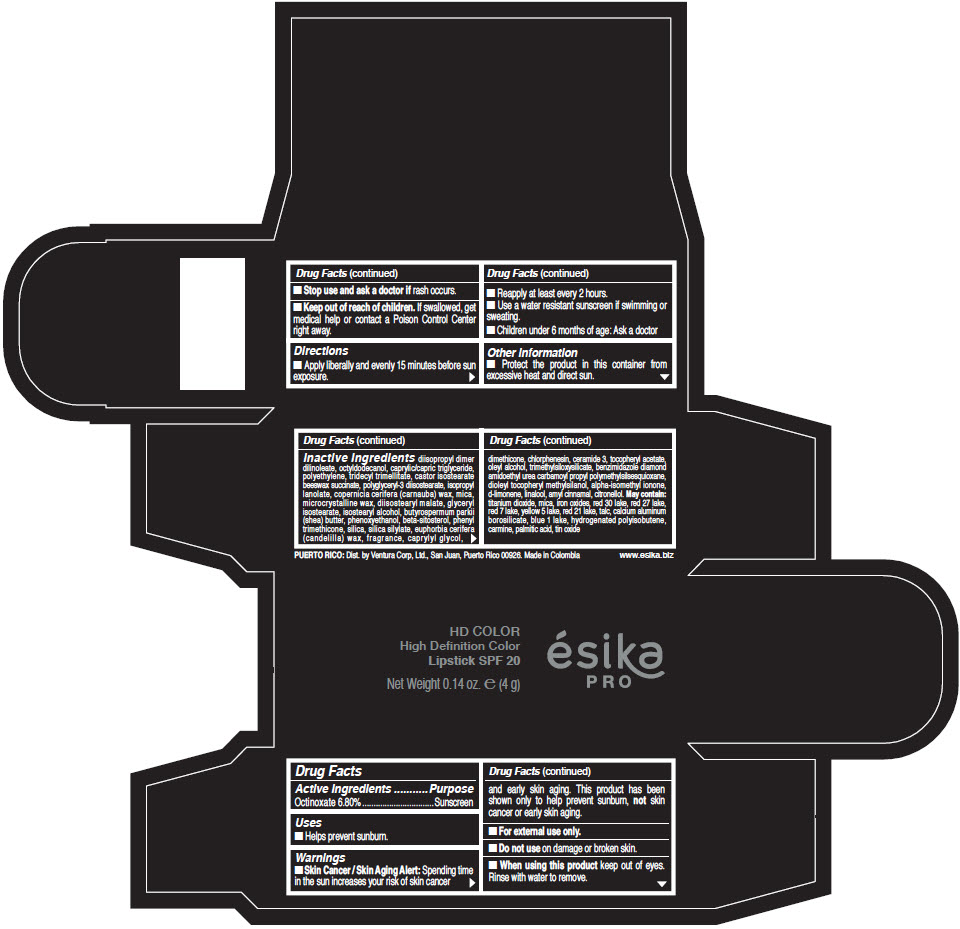
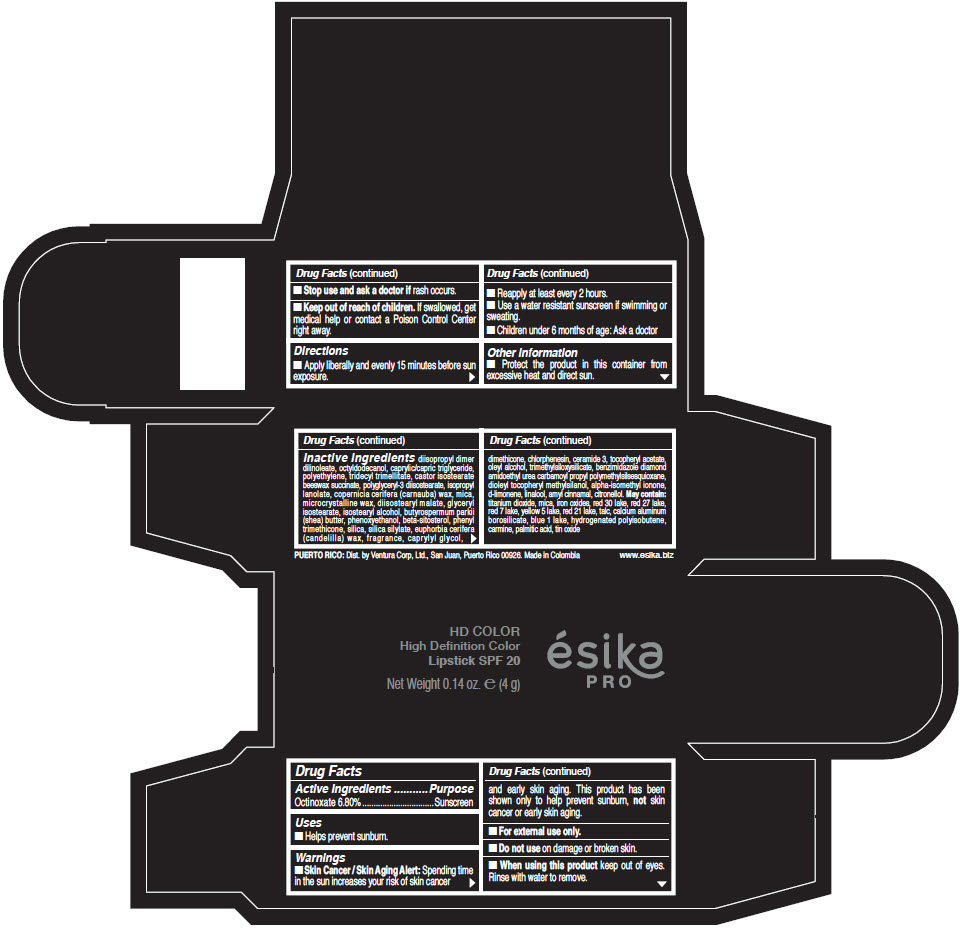
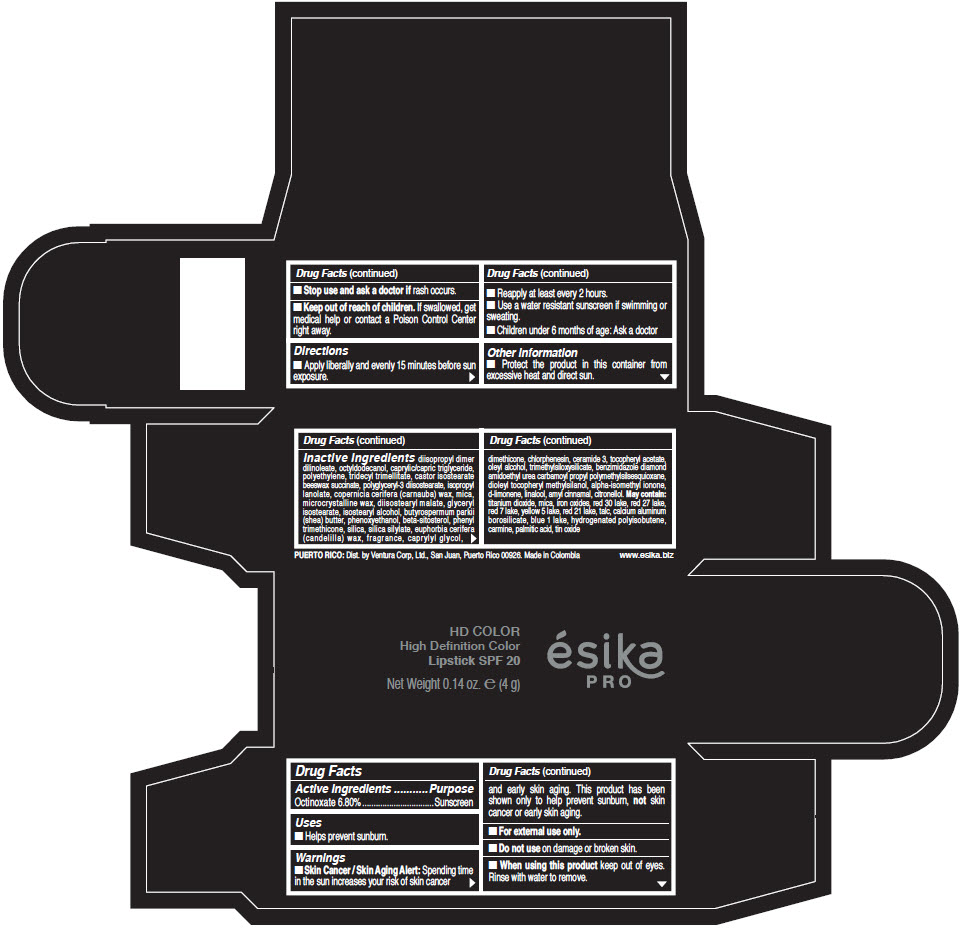
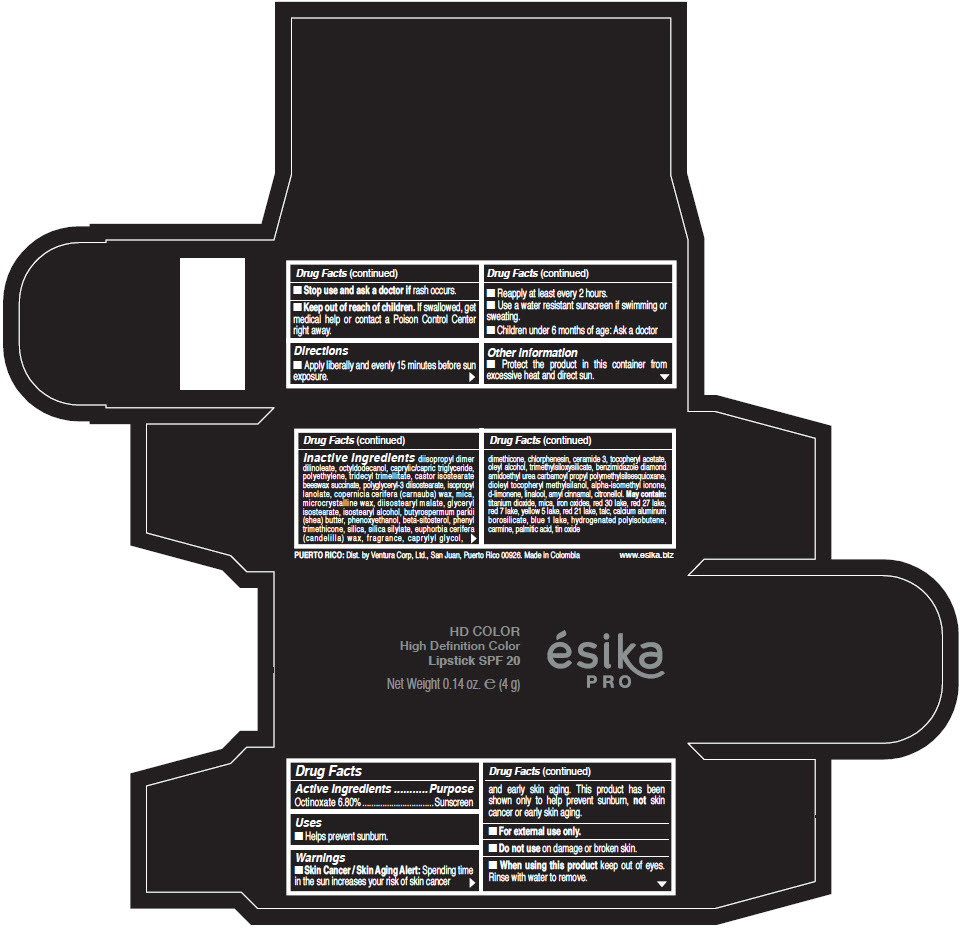
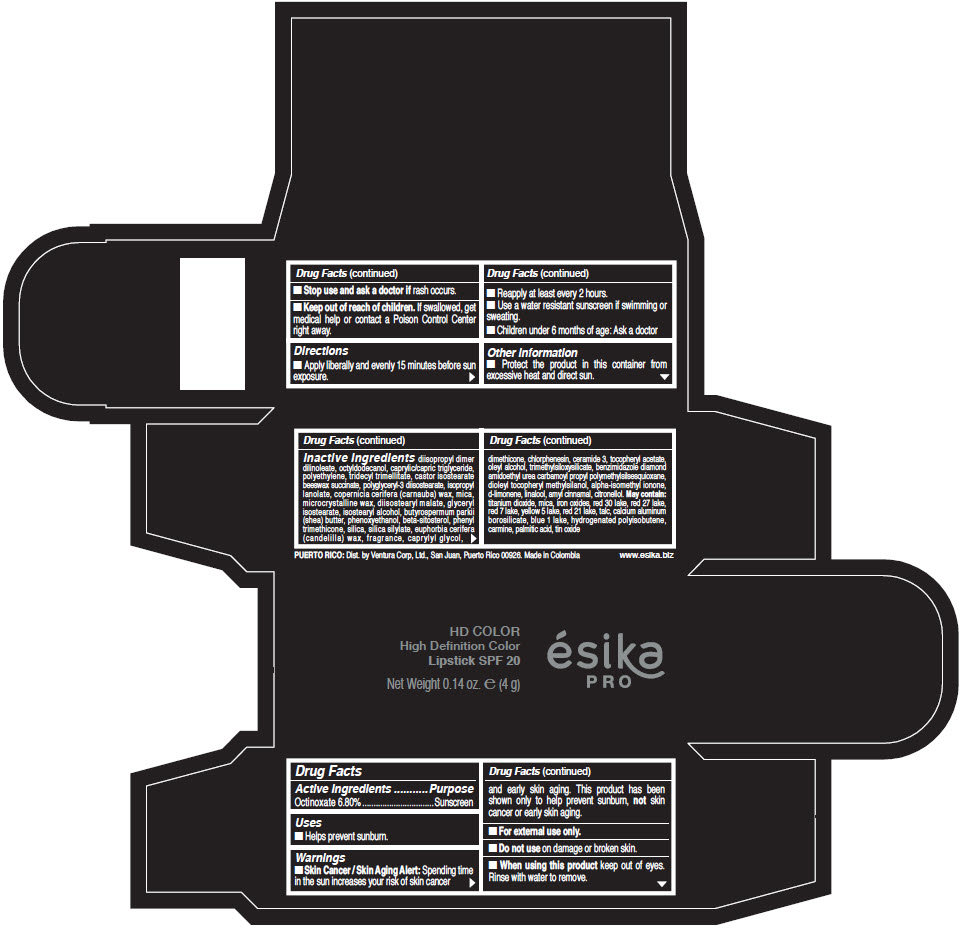
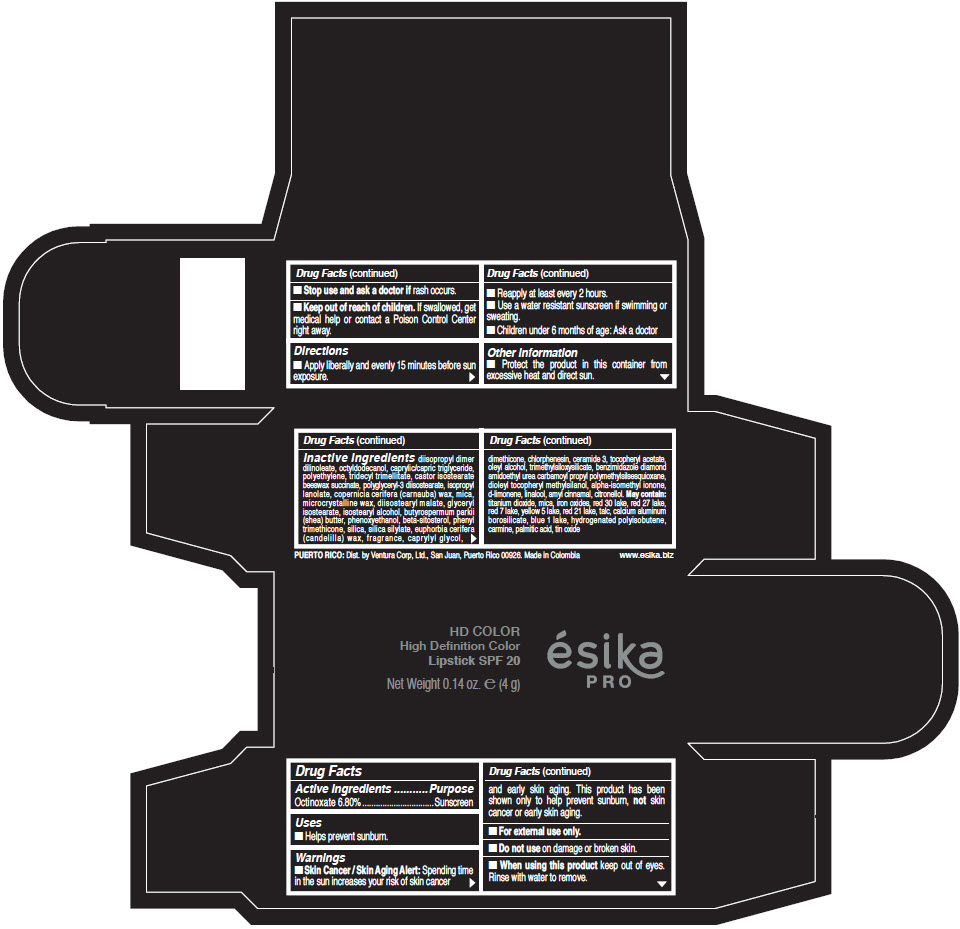
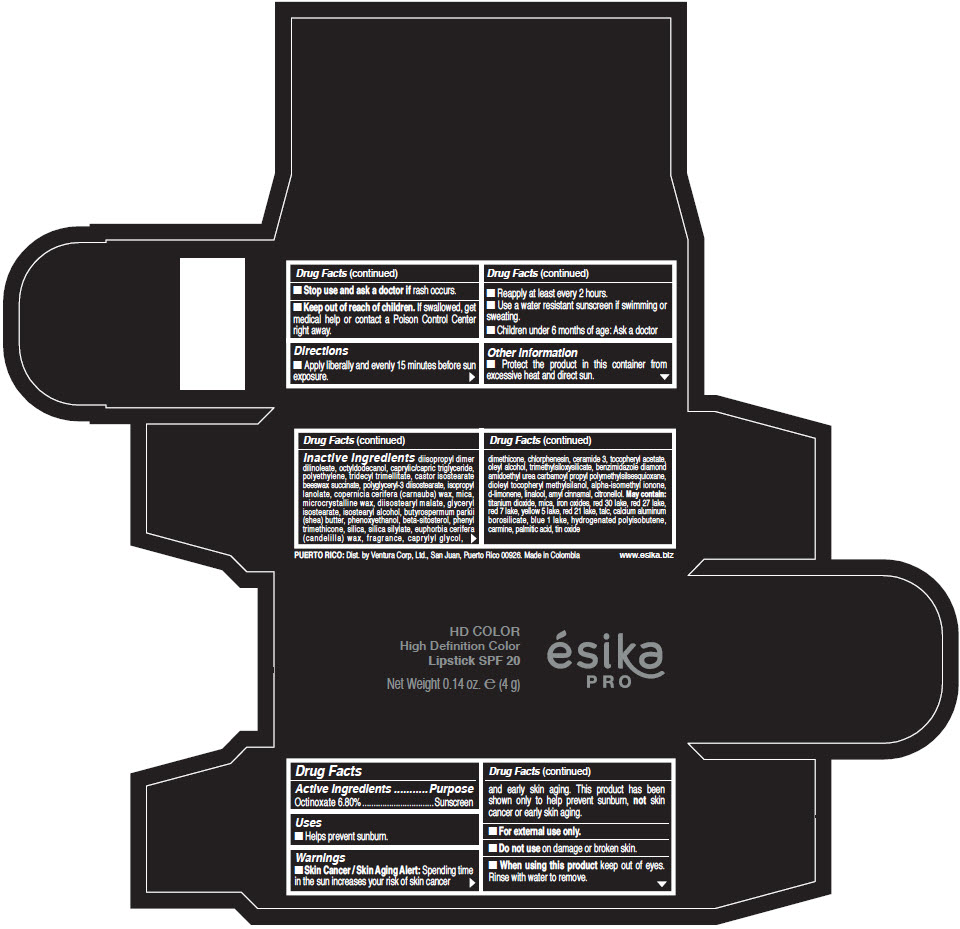
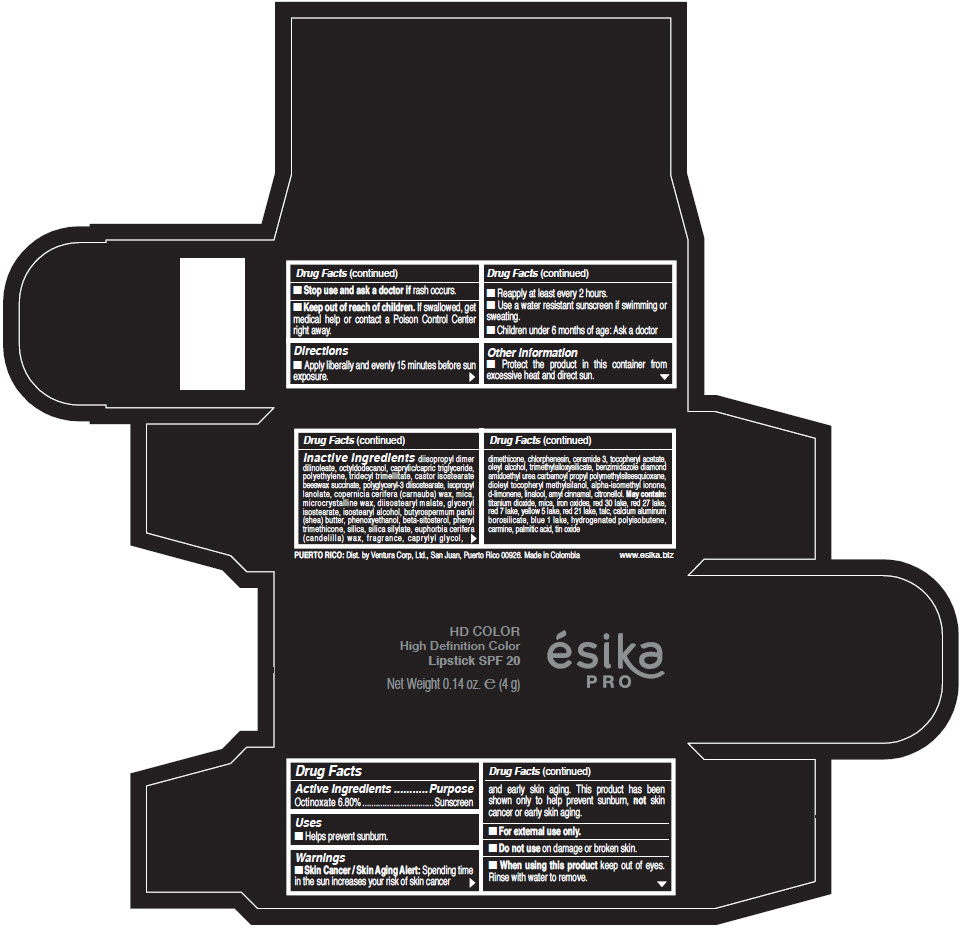
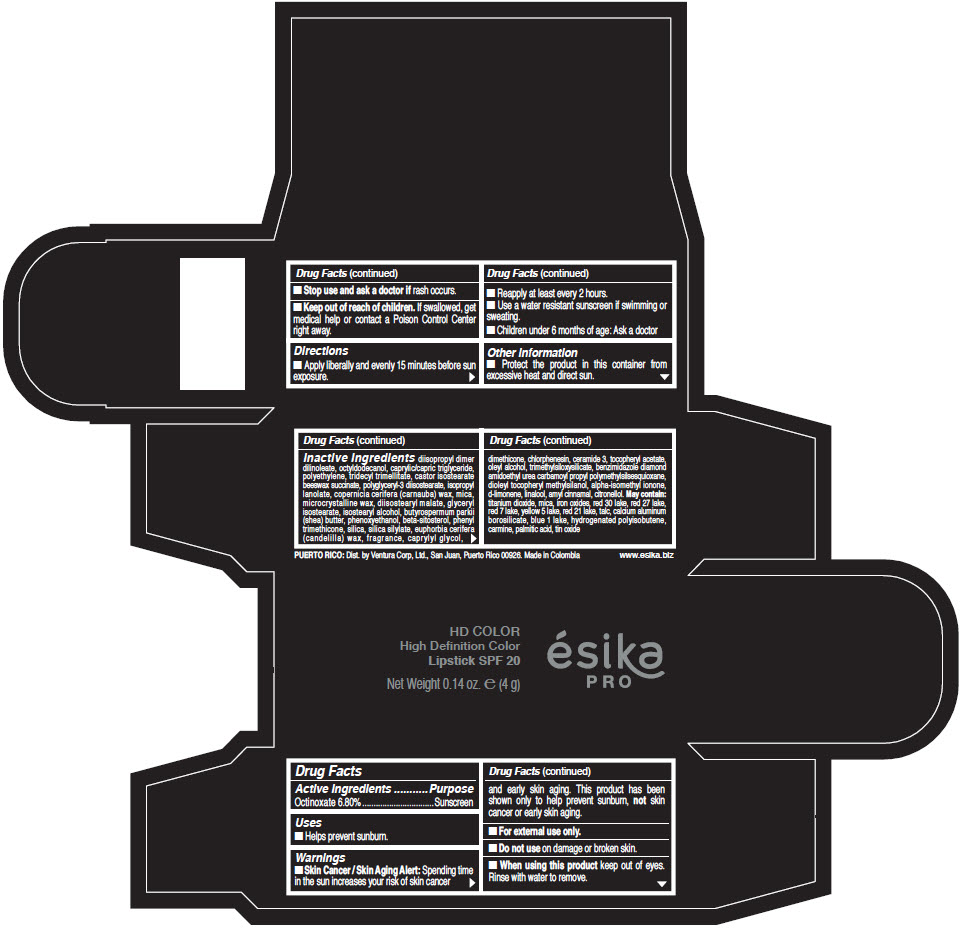
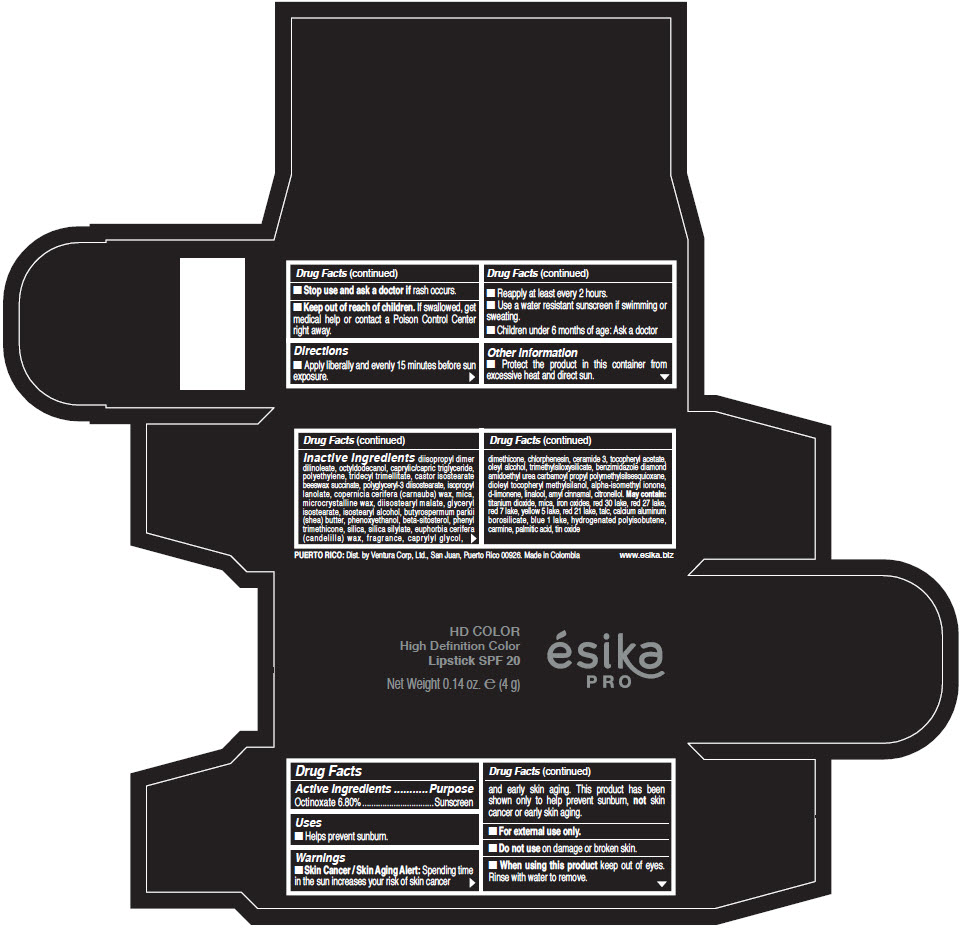
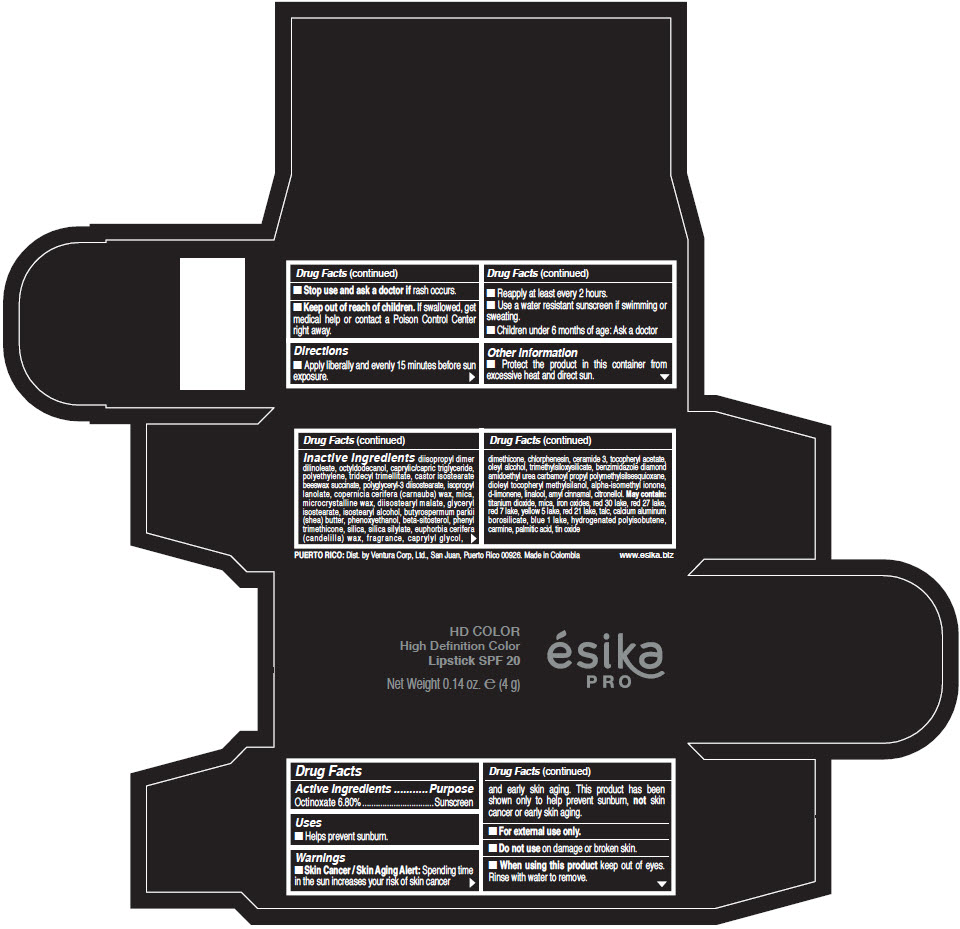
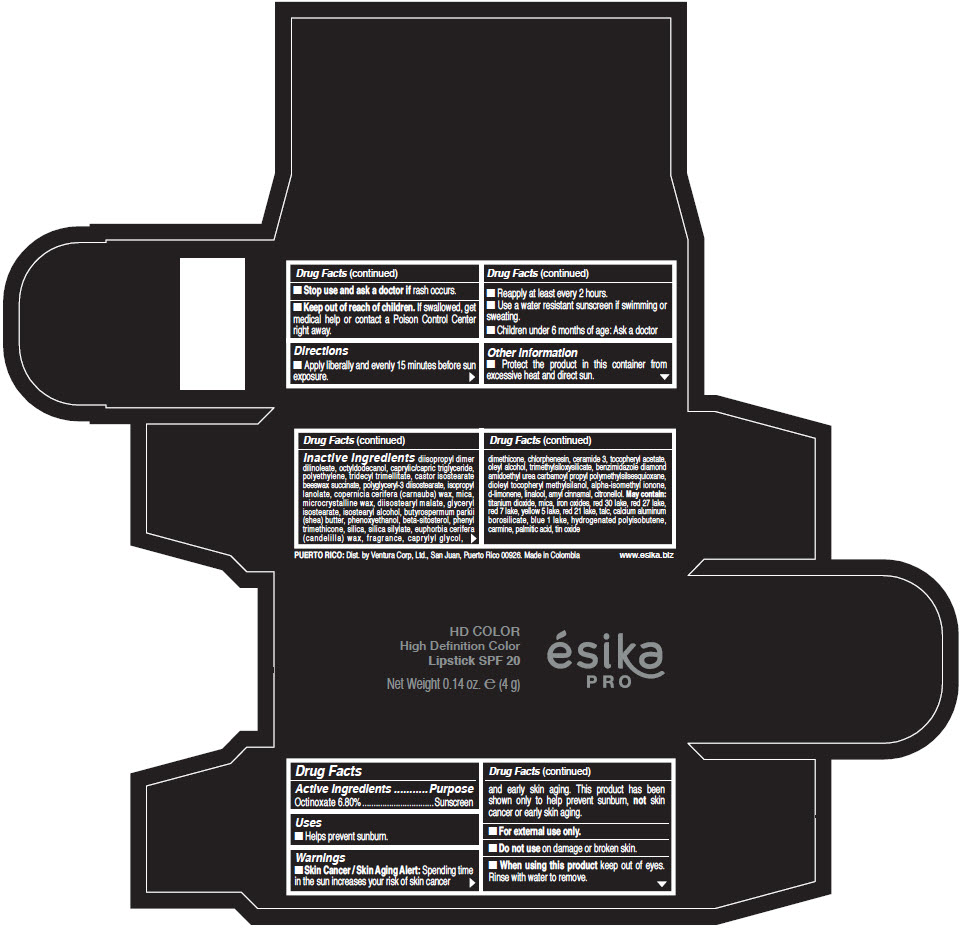
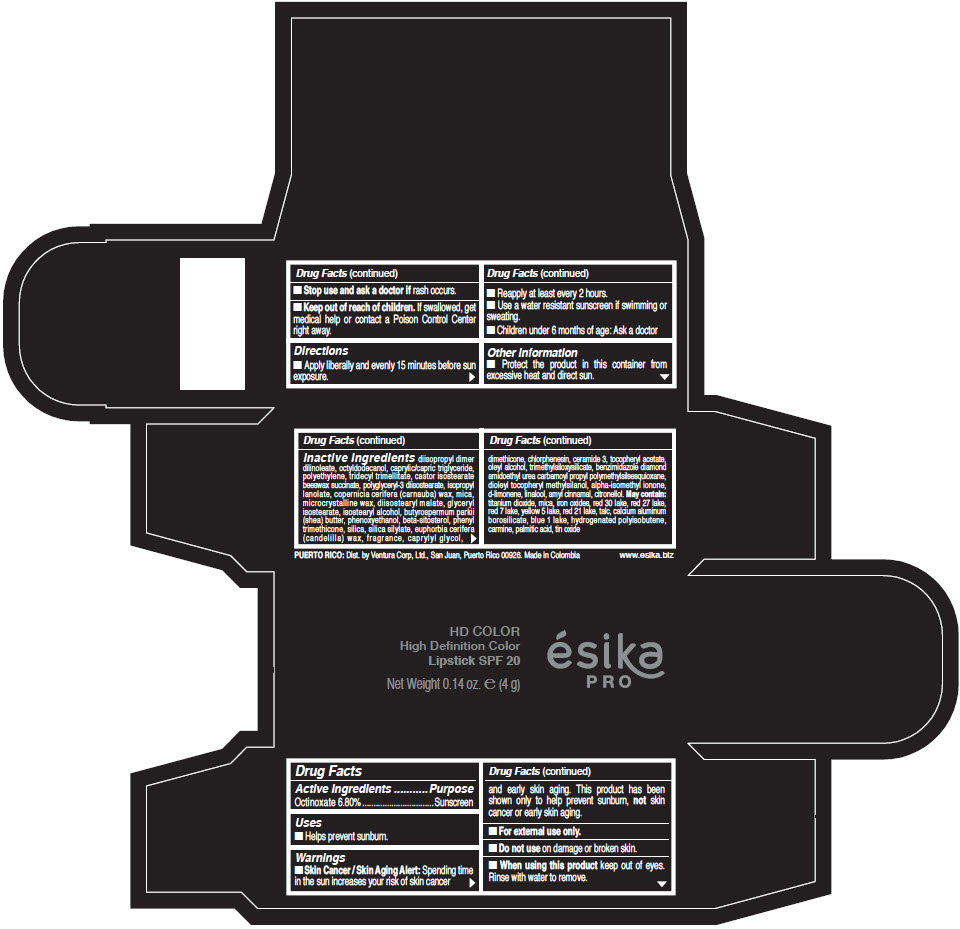
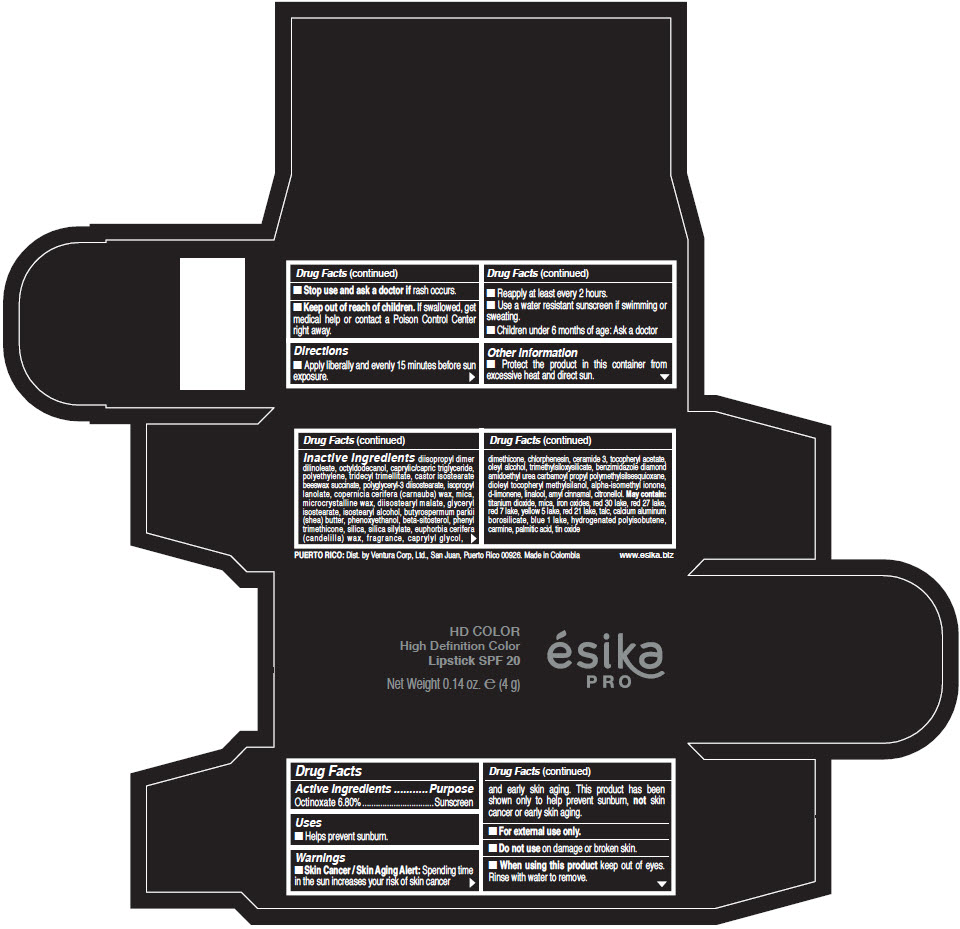
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
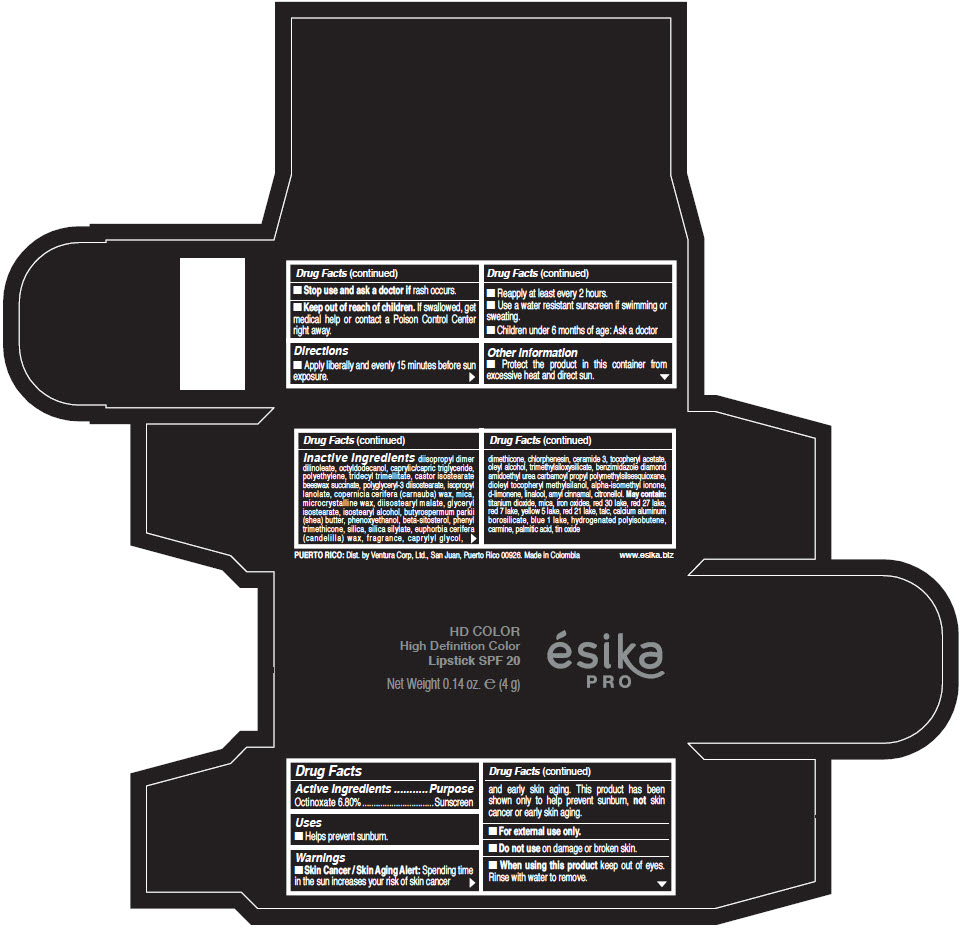
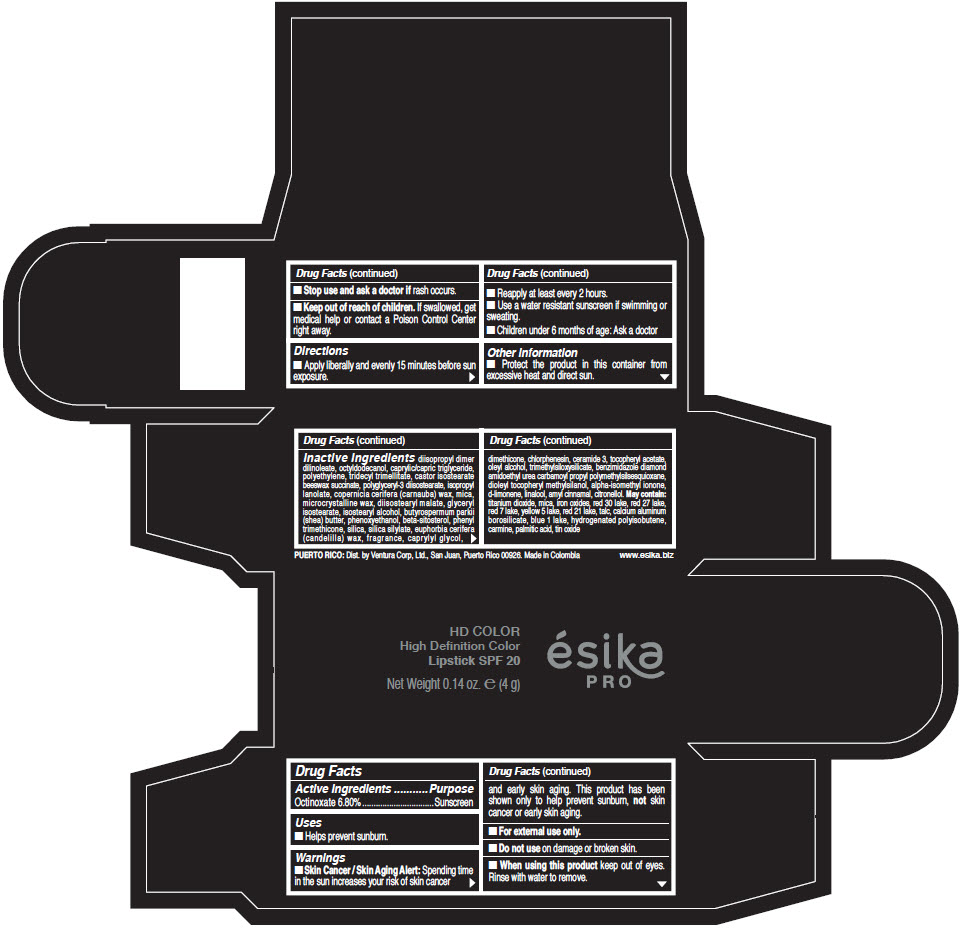
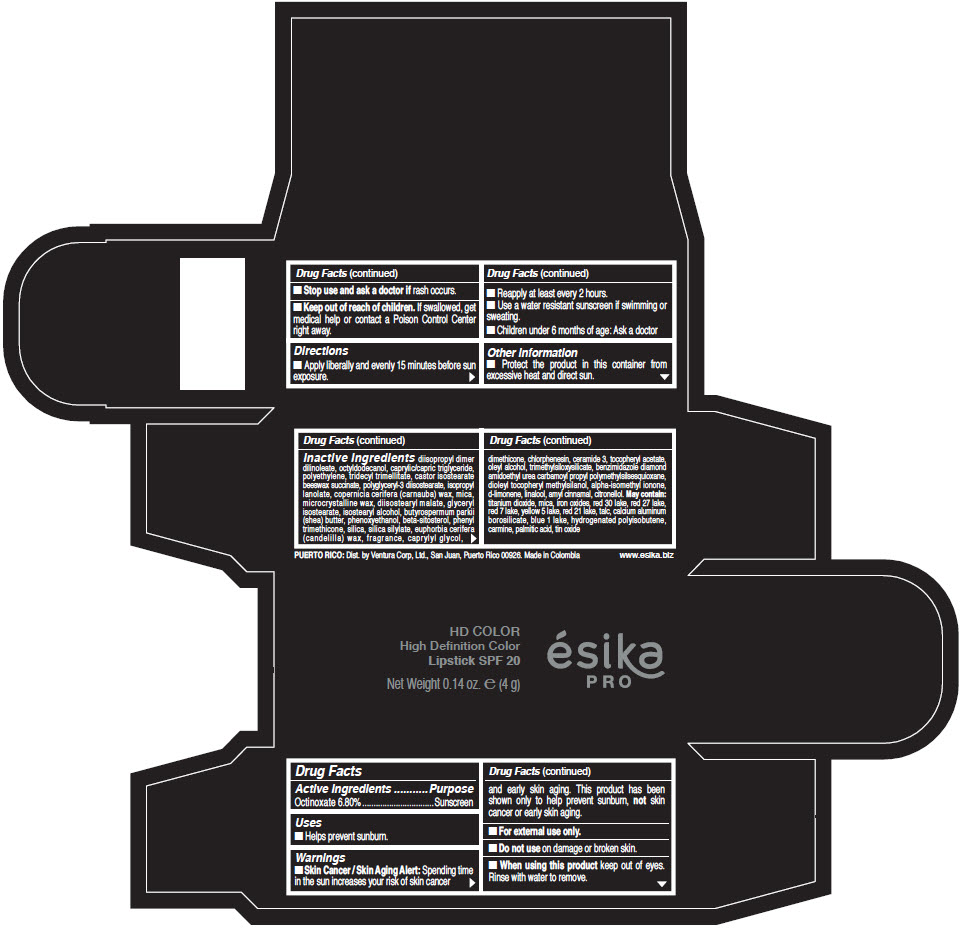
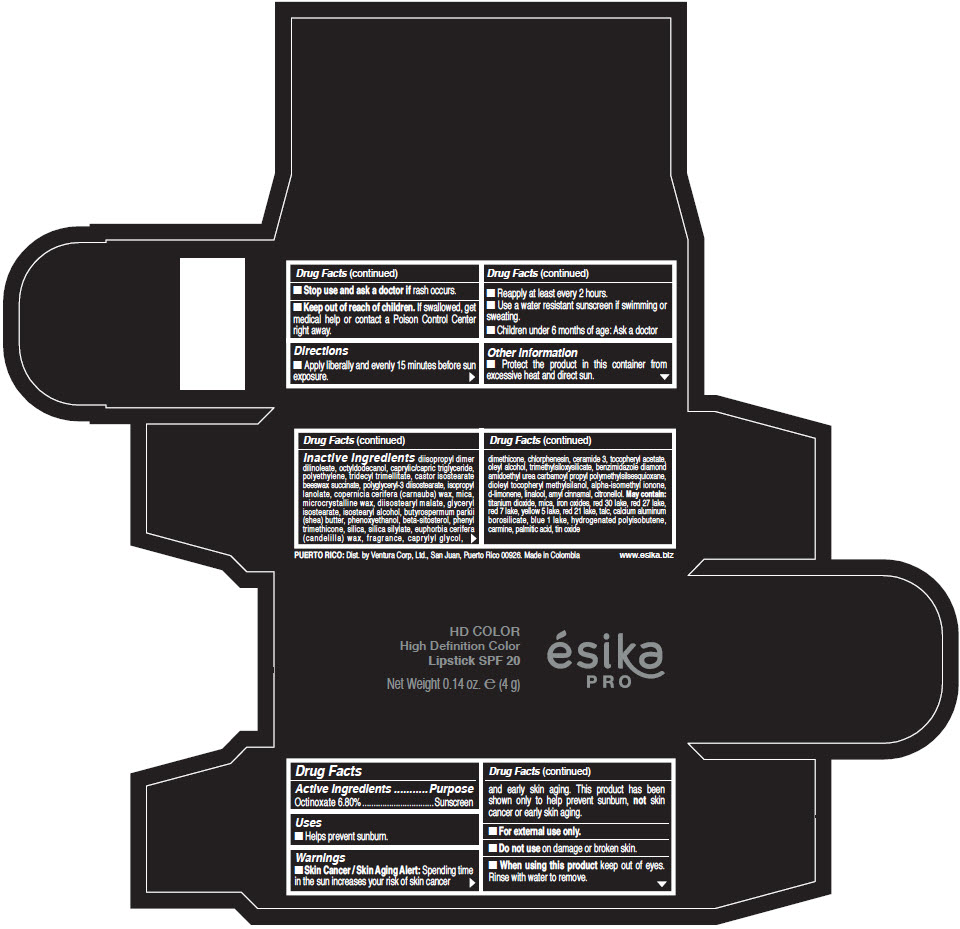
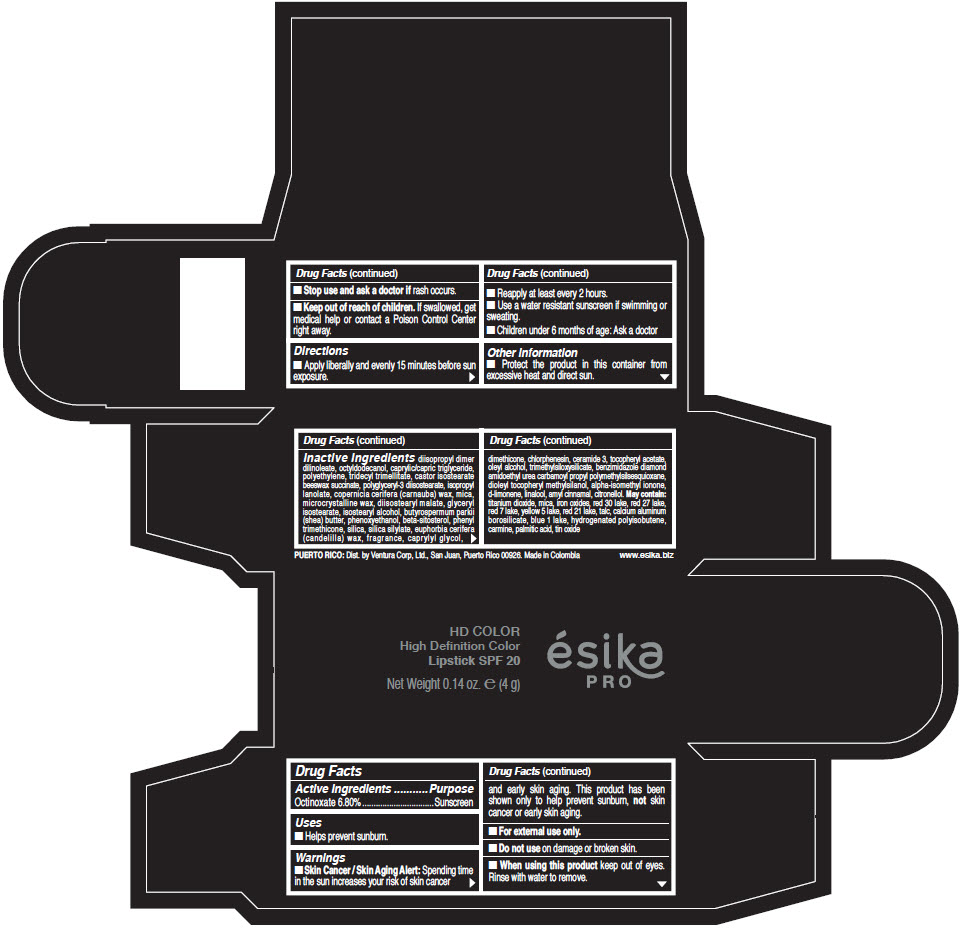
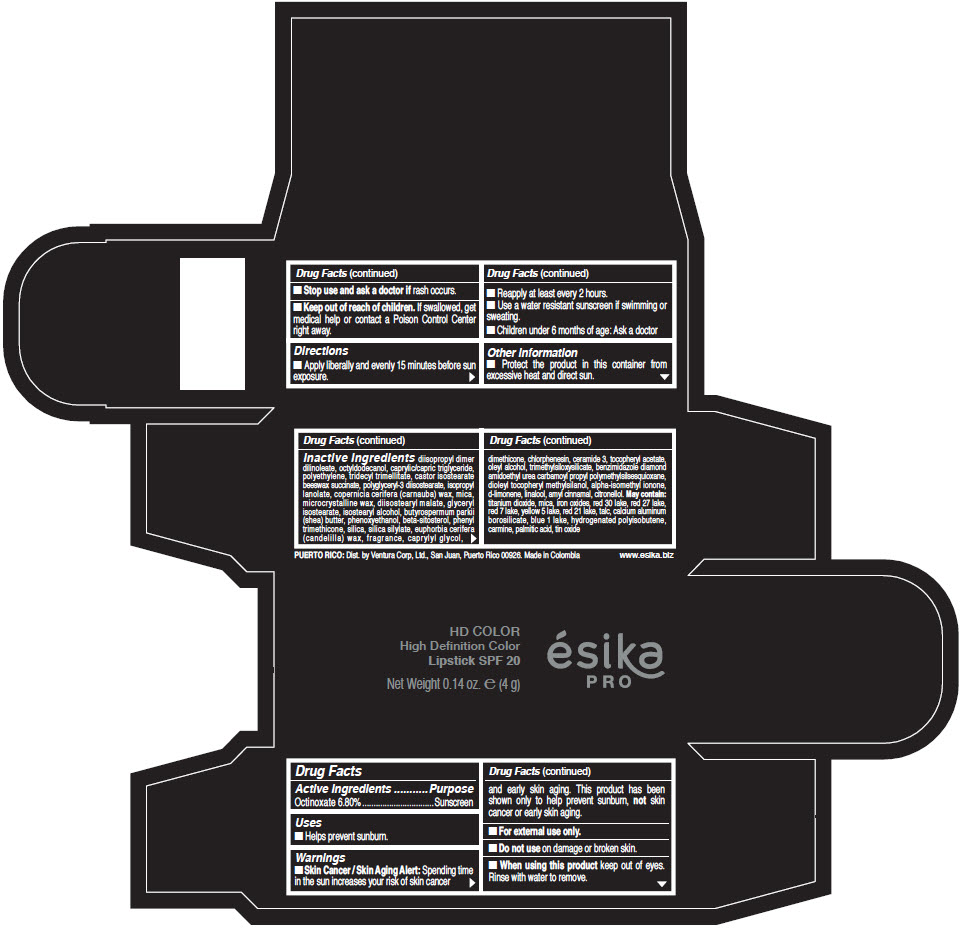
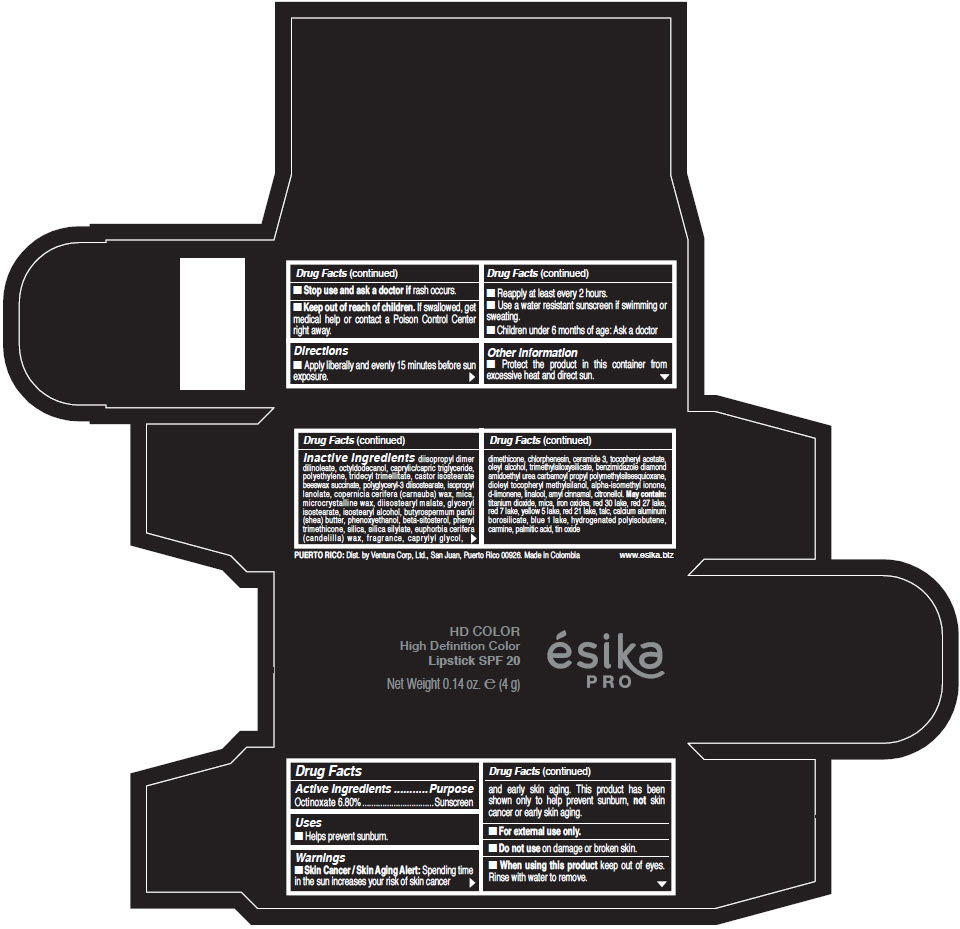
Inactive Ingredients
diisopropyl dimer dilinoleate, octyldodecanol, caprylic/capric triglyceride, polyethylene, tridecyl trimellitate, castor isostearate beeswax succinate, polyglyceryl-3 diisostearate, isopropyl lanolate, copernicia cerifera (carnauba) wax, mica, microcrystalline wax, diisostearyl malate, glyceryl isostearate, isostearyl alcohol, butyrospermum parkii (shea) butter, phenoxyethanol, beta-sitosterol, phenyl trimethicone, silica, silica silylate, euphorbia cerifera (candelilla) wax, fragrance, caprylyl glycol, dimethicone, chlorphenesin, ceramide 3, tocopheryl acetate, oleyl alcohol, trimethylsiloxysilicate, benzimidazole diamond amidoethyl urea carbamoyl propyl polymethylsilsesquioxane, dioleyl tocopheryl methylsilanol, alpha-isomethyl ionone, d-limonene, linalool, amyl cinnamal, citronellol. May contain: titanium dioxide, mica, iron oxides, red 30 lake, red 27 lake, red 7 lake, yellow 5 lake, red 21 lake, talc, calcium aluminum borosilicate, blue 1 lake, hydrogenated polyisobutene, carmine, palmitic acid, tin oxide
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD PIMIENTA CALIENTE) - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD VINO CAUTIVANTE) - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD ROSA VIVA) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD CORAL ENSUENO) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD ROJO GLAM) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD CAFE HABANO) - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD FUCSIA VIBRANTE) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD MELON INTENSE) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD ROSADO CHIC) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD CIRUELA GLAM) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD FRAMBUESA ENCANTO) - PURPLE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD MAGENTA SWEET) - PURPLE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD LAVA LIPS) - ORANGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD FRESA IMPERIAL) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD FUCSIA OPULENTO) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD BERRY BALCANIC) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD ROSA OBSESSION) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (HD ROJO EXHUBERANTE) - RED
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD PIMIENTA CALIENTE) - BROWN
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-729 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-729-02 1 in 1 BOX 02/29/2016 1 NDC:13537-729-01 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD VINO CAUTIVANTE) - BROWN
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-730 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-730-04 1 in 1 BOX 02/29/2016 1 NDC:13537-730-03 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROSA VIVA) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-731 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-731-06 1 in 1 BOX 02/29/2016 1 NDC:13537-731-05 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD CORAL ENSUENO) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-732 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-732-08 1 in 1 BOX 02/29/2016 1 NDC:13537-732-07 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROJO GLAM) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-733 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-733-10 1 in 1 BOX 02/29/2016 1 NDC:13537-733-09 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD CAFE HABANO) - BROWN
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-734 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-734-12 1 in 1 BOX 02/29/2016 1 NDC:13537-734-11 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD FUCSIA VIBRANTE) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-735 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-735-14 1 in 1 BOX 02/29/2016 1 NDC:13537-735-13 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD MELON INTENSE) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-736 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-736-16 1 in 1 BOX 02/29/2016 1 NDC:13537-736-15 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROSADO CHIC) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-737 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-737-18 1 in 1 BOX 02/29/2016 1 NDC:13537-737-17 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD CIRUELA GLAM) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-738 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-738-20 1 in 1 BOX 02/29/2016 1 NDC:13537-738-19 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD FRAMBUESA ENCANTO) - PURPLE
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-739 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-739-22 1 in 1 BOX 02/29/2016 1 NDC:13537-739-21 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD MAGENTA SWEET) - PURPLE
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-740 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-740-24 1 in 1 BOX 02/29/2016 1 NDC:13537-740-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD LAVA LIPS) - ORANGE
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-741 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-741-24 1 in 1 BOX 02/29/2016 1 NDC:13537-741-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD FRESA IMPERIAL) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-742 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-742-24 1 in 1 BOX 02/29/2016 1 NDC:13537-742-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD FUCSIA OPULENTO) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-743 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-743-24 1 in 1 BOX 02/29/2016 1 NDC:13537-743-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD BERRY BALCANIC) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-744 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-744-24 1 in 1 BOX 02/29/2016 1 NDC:13537-744-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROSA OBSESSION) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-745 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-745-24 1 in 1 BOX 02/29/2016 1 NDC:13537-745-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROJO EXHUBERANTE) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-746 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-746-24 1 in 1 BOX 02/29/2016 1 NDC:13537-746-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 02/29/2016 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20
octinoxate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-747 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-747-28 1 in 1 KIT 02/29/2016 12/01/2018 1 NDC:13537-747-27 1 in 1 BOX Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 0.43 g Part 2 1 TUBE 0.43 g Part 3 1 TUBE 0.43 g Part 4 1 TUBE 0.43 g Part 5 1 TUBE 0.43 g Part 6 1 TUBE 0.43 g Part 7 1 TUBE 0.43 g Part 8 1 TUBE 0.43 g Part 9 1 TUBE 0.43 g Part 10 1 TUBE 0.43 g Part 11 1 TUBE 0.43 g Part 12 1 TUBE 0.43 g Part 13 1 TUBE 0.43 g Part 14 1 TUBE 0.43 g Part 15 1 TUBE 0.43 g Part 16 1 TUBE 0.43 g Part 17 1 TUBE 0.43 g Part 18 1 TUBE 0.43 g Part 1 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD PIMIENTA CALIENTE) - BROWN
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 2 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD VINO CAUTIVANTE) - BROWN
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 3 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROSA VIVA) - PINK
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 4 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD CORAL ENSUENO) - RED
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 5 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROJO GLAM) - RED
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 6 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD CAFE HABANO) - BROWN
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 7 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD FUCSIA VIBRANTE) - PINK
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 8 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD MELON INTENSE) - PINK
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 9 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROSADO CHIC) - PINK
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 10 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD CIRUELA GLAM) - RED
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 11 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD FRAMBUESA ENCANTO) - PURPLE
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 12 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD MAGENTA SWEET) - PURPLE
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 13 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD LAVA LIPS) - ORANGE
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 14 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD FRESA IMPERIAL) - RED
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 15 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD FUCSIA OPULENTO) - PINK
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 16 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD BERRY BALCANIC) - RED
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 17 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROSA OBSESSION) - PINK
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Part 18 of 18 ESIKA PRO HD COLOR HIGH DEFINITION COLOR SPF 20 (HD ROJO EXHUBERANTE) - RED
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) OCTYLDODECANOL (UNII: 461N1O614Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) PALMITIC ACID (UNII: 2V16EO95H1) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.43 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/29/2016 12/01/2018 Labeler - Ventura Corporation LTD (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-729, 13537-730, 13537-731, 13537-732, 13537-733, 13537-734, 13537-735, 13537-736, 13537-737, 13537-738, 13537-739, 13537-740, 13537-741, 13537-742, 13537-743, 13537-744, 13537-745, 13537-746, 13537-747)