Label: KOLORZ SIXTY SECOND FLUORIDE CHERRY CHEESECAKE- sodium fluoride gel
KOLORZ SIXTY SECOND FLUORIDE PINA COLADA- sodium fluoride gel
KOLORZ SIXTY SECOND FLUORIDE TRIPLE MINT- sodium fluoride gel
KOLORZ SIXTY SECOND FLUORIDE BLUE RASPBERRY- sodium fluoride gel
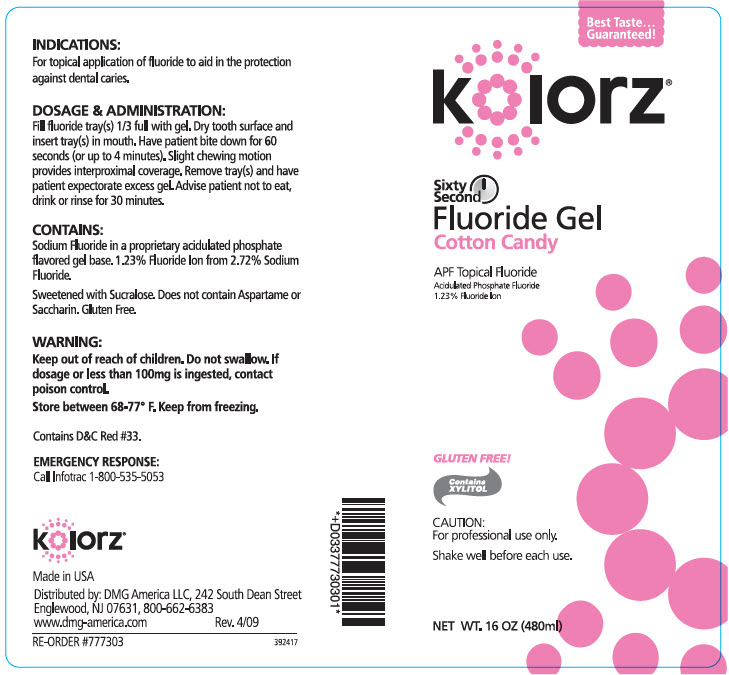
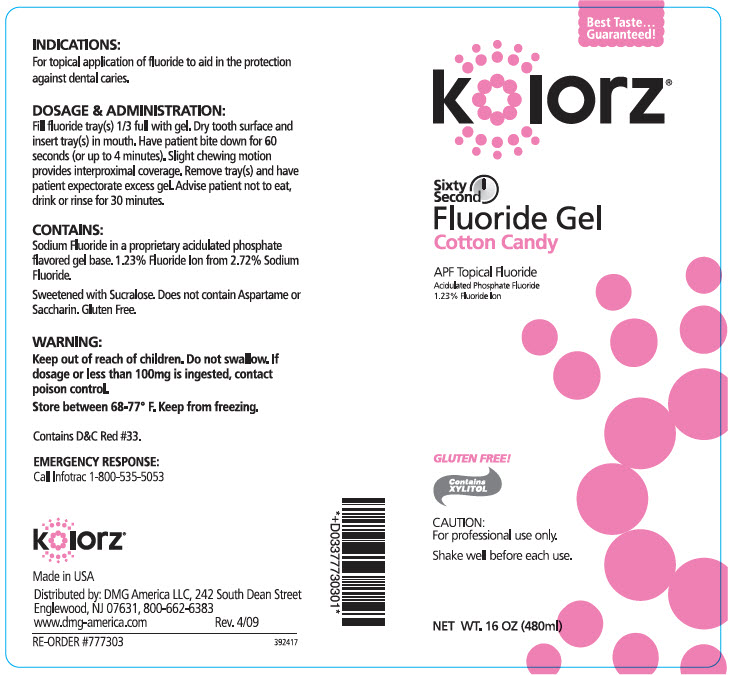
KOLORZ SIXTY SECOND FLUORIDE COTTON CANDY- sodium fluoride gel
-
NDC Code(s):
25047-771-16,
25047-772-16,
25047-773-16,
25047-774-16, view more25047-775-16
- Packager: DMG AMERICA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 28, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS
-
DOSAGE & ADMINISTRATION
Fill fluoride tray(s) 1/3 full with gel. Dry tooth surface and insert tray(s) in mouth. Have patient bite down for 60 seconds (or up to 4 minutes). Slight chewing motion provides interproximal coverage. Remove tray(s) and have patient expectorate excess gel. Advise patient not to eat, drink or rinse for 30 minutes.
- CONTAINS
- WARNING
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- EMERGENCY RESPONSE
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Cherry Cheesecake
- PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Piña Colada
- PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Triple Mint
- PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Blue Raspberry
- PRINCIPAL DISPLAY PANEL - 480 ml Bottle Label - Cotton Candy
-
INGREDIENTS AND APPEARANCE
KOLORZ SIXTY SECOND FLUORIDE CHERRY CHEESECAKE
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-772 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 12.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor CHERRY (CHERRY CHEESECAKE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-772-16 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 KOLORZ SIXTY SECOND FLUORIDE PINA COLADA
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-775 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 12.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor PINEAPPLE (PIÑA COLADA) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-775-16 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 KOLORZ SIXTY SECOND FLUORIDE TRIPLE MINT
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-771 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 12.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor MINT (TRIPLE MINT) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-771-16 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 KOLORZ SIXTY SECOND FLUORIDE BLUE RASPBERRY
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-774 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 12.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor RASPBERRY (BLUE RASPBERRY) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-774-16 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 KOLORZ SIXTY SECOND FLUORIDE COTTON CANDY
sodium fluoride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25047-773 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 12.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color Score Shape Size Flavor COTTON CANDY (COTTON CANDY) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25047-773-16 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/22/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/22/2006 Labeler - DMG AMERICA, LLC (106792427) Establishment Name Address ID/FEI Business Operations KEYSTONE INDUSTRIES 014769301 MANUFACTURE(25047-771, 25047-772, 25047-773, 25047-774, 25047-775)