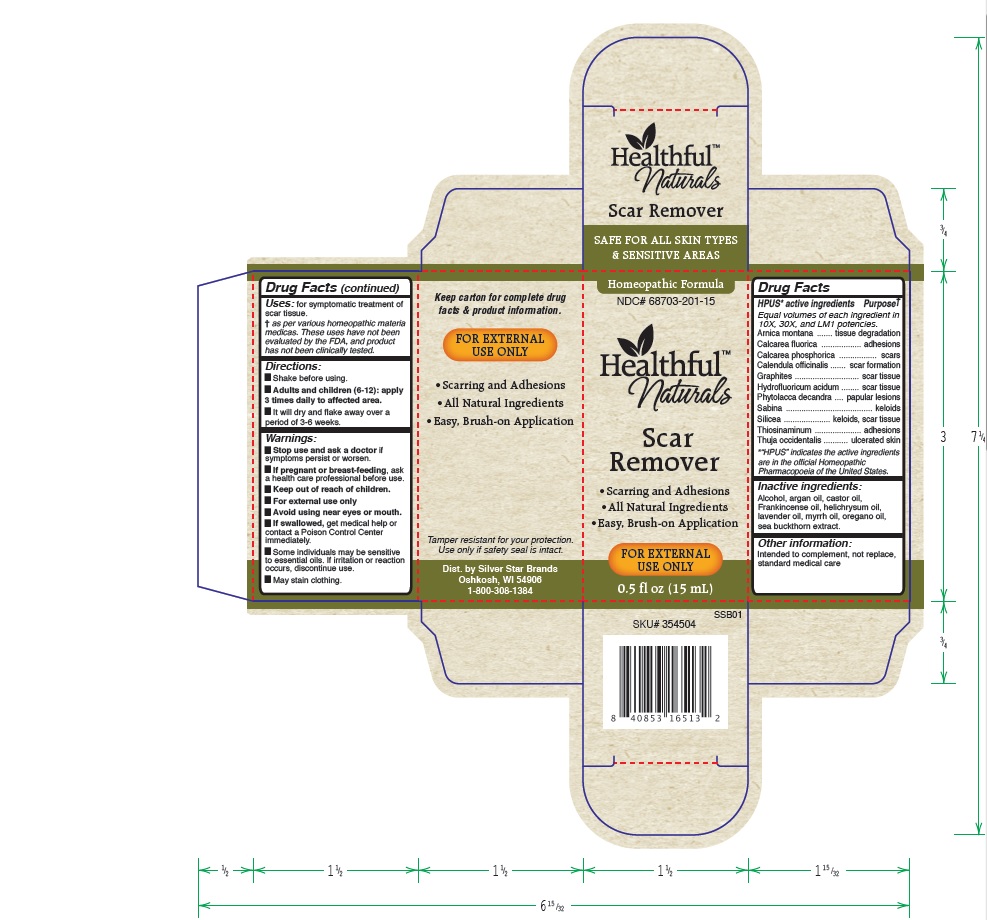
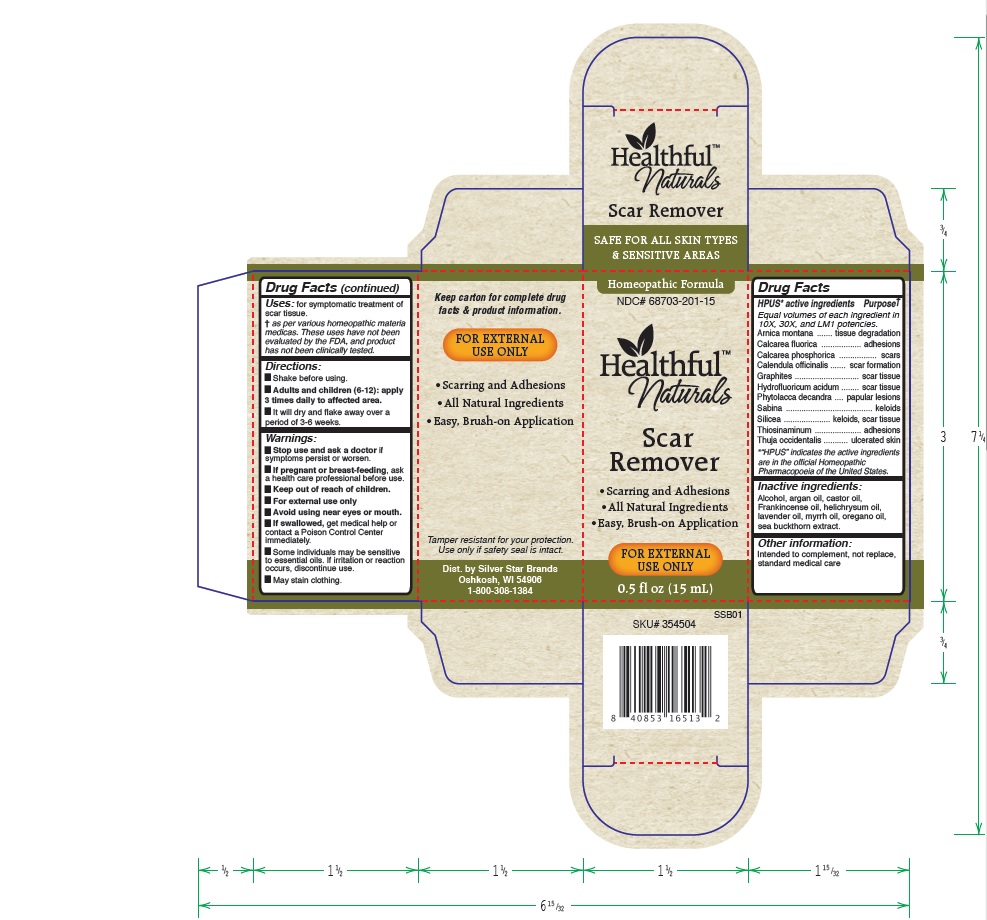
Label: HEALTHFUL NATURALS SCAR REMOVER- arnica montana, calcarea fluorica, calcarea phosphorica, calendula officinalis, graphites, hydrofluoricum acidum, phytolacca decandra, sabina, silicea, thiosinaminum, thuja occidentalis liquid liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 68703-201-15 - Packager: Silver Star Brands
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 12, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- HPUS active ingredients:
- Uses
-
Warnings
Stop use and ask your doctor if symptoms persist or worsen.
If pregnant or breast-feeding, ask a healthcare professional before use.
Keep out of reach of children.
For external use only.
Avoid using near eyes or mouth.
If swallowed, get medical help or contact a Poison Control Center immediately.
Some individuals may be sensitive to essential oils. If irritation or reaction occurs, discontinue use.
May stain clothing.
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information:
- Inactive ingredients:
-
HPUS active ingredients purpose
Equal volumes of each ingredient in 10X, 30X, and LM1 potencies.
Arnica montana......................................................tissue degradation
Calcarea fluorica.....................................................adhesions
Calcarea phosphorica...............................................scars
Calendula officinalis.................................................scar formation
Graphites...............................................................scar tissue
Hydrofluoricum acidum............................................scar tissue
Phytolacca decandra................................................papular lesions
Sabina...................................................................keloids
Silicea....................................................................keloids, scar tissue
Thiosinaminum........................................................adhesions
Thuja occidentalis....................................................ulcerated skin
"HPUS" indicates the active ingredients are in the official Homeopathic Pharmacopeia of the United States
- Content of Labeling
-
INGREDIENTS AND APPEARANCE
HEALTHFUL NATURALS SCAR REMOVER
arnica montana, calcarea fluorica, calcarea phosphorica, calendula officinalis, graphites, hydrofluoricum acidum, phytolacca decandra, sabina, silicea, thiosinaminum, thuja occidentalis liquid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68703-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (PHOSPHATE ION - UNII:NK08V8K8HR) TRIBASIC CALCIUM PHOSPHATE 10 [hp_X] in 15 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 10 [hp_X] in 15 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 10 [hp_X] in 15 mL ALLYLTHIOUREA (UNII: 706IDJ14B7) (ALLYLTHIOUREA - UNII:706IDJ14B7) ALLYLTHIOUREA 10 [hp_X] in 15 mL HYDROFLUORIC ACID (UNII: RGL5YE86CZ) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 10 [hp_X] in 15 mL ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 10 [hp_X] in 15 mL CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 10 [hp_X] in 15 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 10 [hp_X] in 15 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 10 [hp_X] in 15 mL JUNIPERUS SABINA LEAFY TWIG (UNII: Z5BEX9K2G1) (JUNIPERUS SABINA LEAFY TWIG - UNII:Z5BEX9K2G1) JUNIPERUS SABINA LEAFY TWIG 10 [hp_X] in 15 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 10 [hp_X] in 15 mL Inactive Ingredients Ingredient Name Strength MYRRH OIL (UNII: H74221J5J4) OREGANO LEAF OIL (UNII: 7D0CGR40U1) HIPPOPHAE RHAMNOIDES FRUIT OIL (UNII: TA4JCF9S1J) HELICHRYSUM ITALICUM FLOWER OIL (UNII: O97ZV7726K) ALCOHOL (UNII: 3K9958V90M) ARGAN OIL (UNII: 4V59G5UW9X) CASTOR OIL (UNII: D5340Y2I9G) FRANKINCENSE OIL (UNII: 67ZYA5T02K) LAVENDER OIL (UNII: ZBP1YXW0H8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68703-201-15 15 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 10/28/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/28/2015 Labeler - Silver Star Brands (006070379) Registrant - Silver Star Brands (006070379)