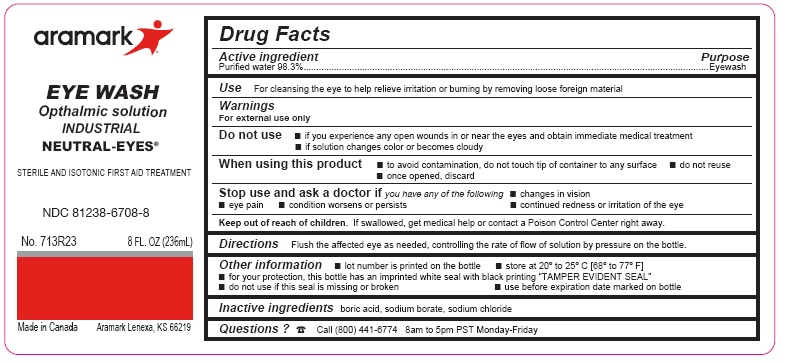
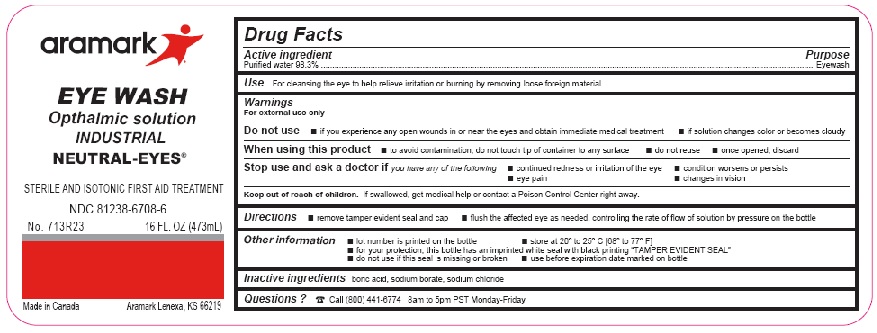
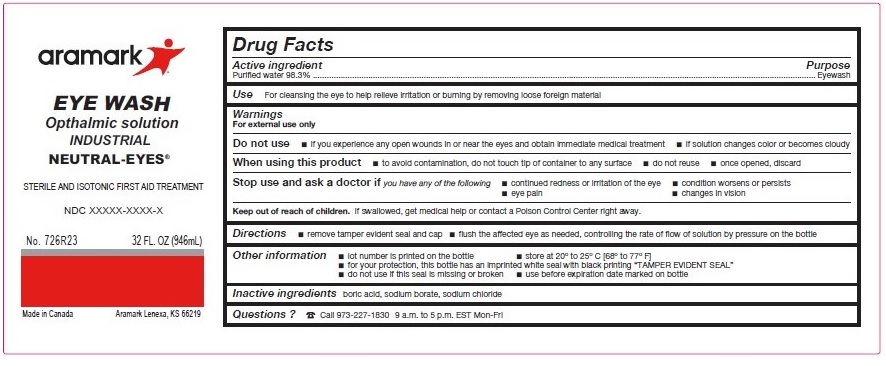
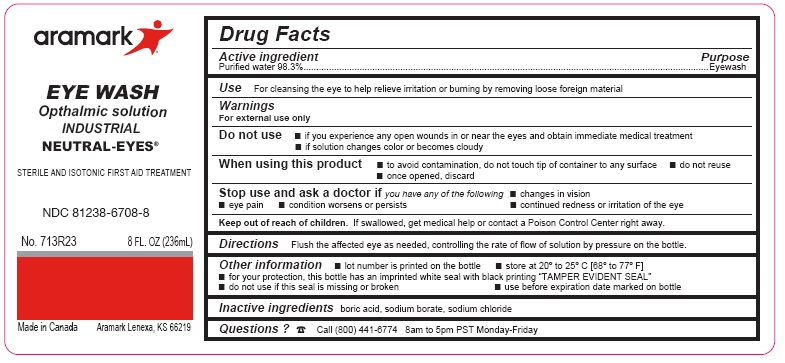
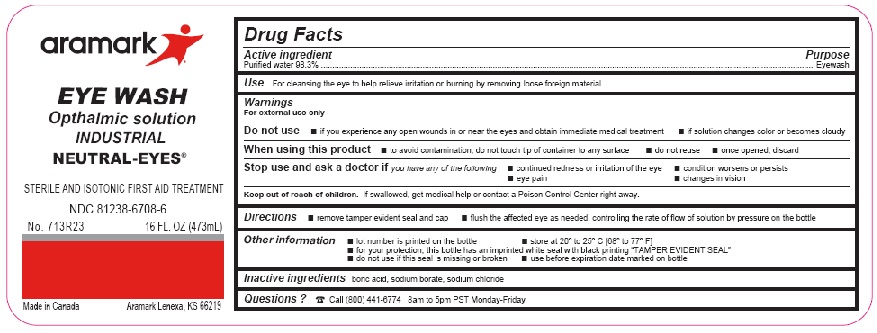
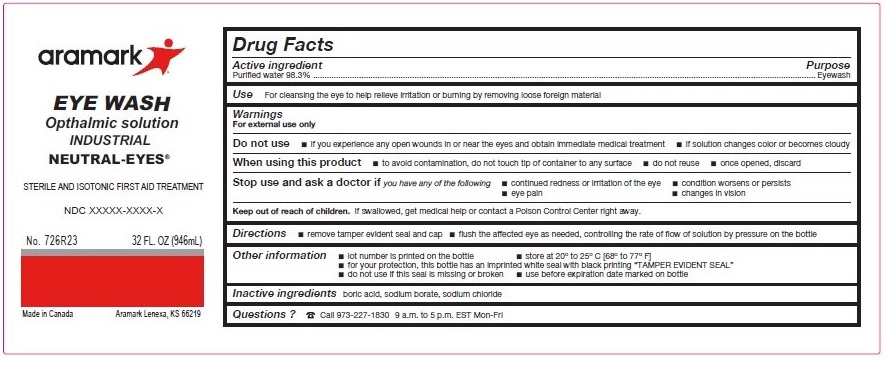
Label: NEUTRAL-EYES- purified water 98.3% solution
-
NDC Code(s):
81238-6708-1,
81238-6708-3,
81238-6708-4,
81238-6708-6, view more81238-6708-8
- Packager: Western First Aid Safety
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 18, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
Do not use■ if you experience any open wounds in or near the eyes
and get medical help right away
■ if solution changes color or becomes cloudyWhen using this product ■ to avoid
contamination, do not touch tip of container to any
surface ■ do not reuse ■ once opened, discardStop use and ask a doctor if you experience
■ eye pain ■ changes in vision ■ continued redness
or irritation of the eye ■ condition worsens or persists - KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INFORMATION FOR PATIENTS
- INACTIVE INGREDIENT
- QUESTIONS
- Product Labeling
-
INGREDIENTS AND APPEARANCE
NEUTRAL-EYES
purified water 98.3% solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81238-6708 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.3 g in 100 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81238-6708-1 30 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 02/15/2021 2 NDC:81238-6708-3 946 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2021 3 NDC:81238-6708-4 118 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 02/15/2021 4 NDC:81238-6708-6 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2021 5 NDC:81238-6708-8 236 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022305 02/15/2021 Labeler - Western First Aid Safety (043861524) Registrant - Western First Aid Safety (043861524) Establishment Name Address ID/FEI Business Operations Niagara Pharmaceuticals, inc. 205477792 manufacture(81238-6708) , label(81238-6708) , pack(81238-6708)