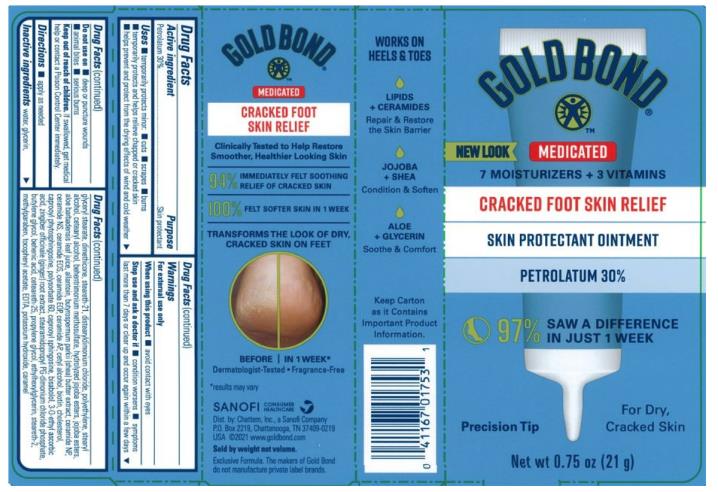
Label: GOLD BOND MEDICATED CRACKED FOOT SKIN RELIEF- petrolatum ointment
- NDC Code(s): 41167-0175-3
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
water, glycerin, glyceryl stearate, dimethicone, steareth-21, distearyldimonium chloride, polyethylene, stearyl alcohol, cetearyl alcohol, behentrimonium methosulfate, hydrolyzed jojoba esters, jojoba esters, aloe barbadensis leaf juice, allantoin, butyrospermum parkii (shea) butter extract, ceramide NP, ceramide NS, ceramide EOS, ceramide EOP, ceramide AP, cocodimonium hydroxypropyl hydrolyzed rice protein, cetyl alcohol, biotin, cholesterol, caprooyl phytosphingosine, polysorbate 60, caprooyl sphingosine, bisabolol, 3-O-ethyl ascorbic acid, zingiber officinale (ginger) root extract, stearamidopropyl PG-dimonium chloride phosphate, butylene glycol, behenic acid, ceteareth-25, propylene glycol, ethylhexylglycerin, steareth-2, methylparaben, tocopheryl acetate, hydrolyzed collagen, EDTA, potassium hydroxide, caramel
Please keep carton as it contains important information.
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GOLD BOND MEDICATED CRACKED FOOT SKIN RELIEF
petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0175 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 30 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) DIMETHICONE (UNII: 92RU3N3Y1O) STEARETH-21 (UNII: 53J3F32P58) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) HYDROLYZED JOJOBA ESTERS (ACID FORM) (UNII: UDR641JW8W) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALLANTOIN (UNII: 344S277G0Z) SHEA BUTTER (UNII: K49155WL9Y) CERAMIDE NP (UNII: 4370DF050B) CERAMIDE NG (UNII: C04977SRJ5) CERAMIDE EOS (UNII: CR0J8RN66K) CERAMIDE 1 (UNII: 5THT33P7X7) CERAMIDE AP (UNII: F1X8L2B00J) COCODIMONIUM HYDROXYPROPYL HYDROLYZED KERATIN (1000 MW) (UNII: 8V0I3U3HMO) CETYL ALCOHOL (UNII: 936JST6JCN) BIOTIN (UNII: 6SO6U10H04) CHOLESTEROL (UNII: 97C5T2UQ7J) POLYSORBATE 60 (UNII: CAL22UVI4M) N-HEXANOYLSPHINGOSINE (UNII: 038753E78J) LEVOMENOL (UNII: 24WE03BX2T) 3-O-ETHYL ASCORBIC ACID (UNII: 6MW60CB71P) GINGER (UNII: C5529G5JPQ) STEARAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: W6000VEI5Y) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BEHENIC ACID (UNII: H390488X0A) CETEARETH-25 (UNII: 8FA93U5T67) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) STEARETH-2 (UNII: V56DFE46J5) METHYLPARABEN (UNII: A2I8C7HI9T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYDROLYSED BOVINE COLLAGEN (ENZYMATIC; 3500 MW) (UNII: 5WE8P977RQ) EDETIC ACID (UNII: 9G34HU7RV0) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) CARAMEL (UNII: T9D99G2B1R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0175-3 1 in 1 CARTON 01/01/2021 1 21 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 01/01/2021 Labeler - Chattem, Inc. (003336013)