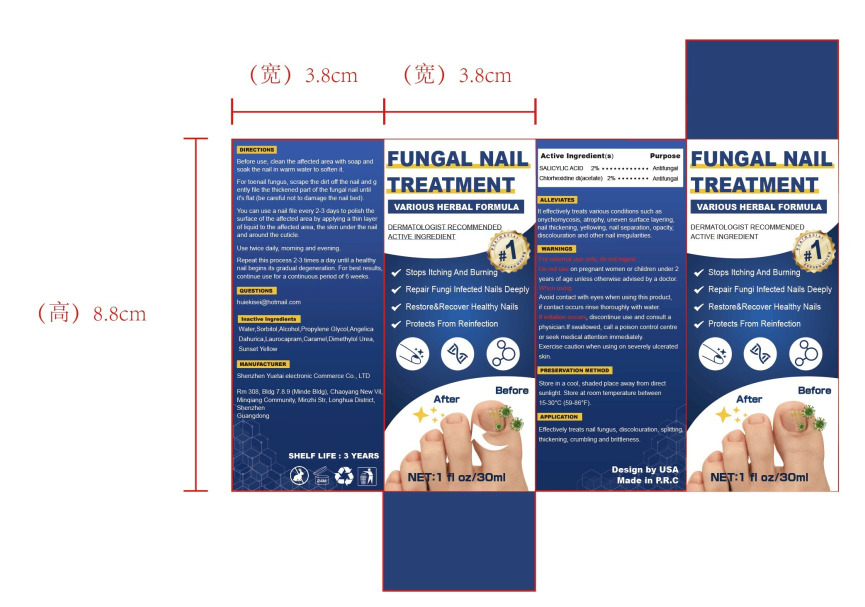
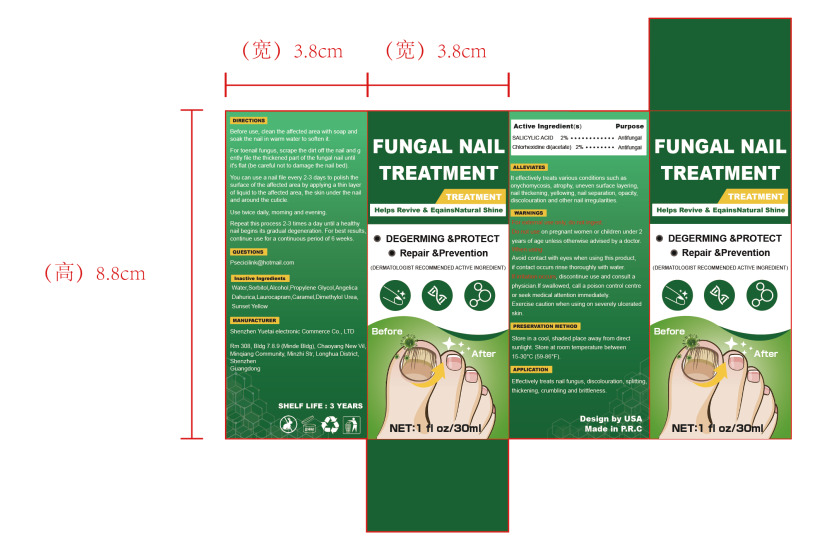
Label: FUNGAL NAIL TREATMENT liquid
- NDC Code(s): 84291-003-01
- Packager: Shenzhen Yuetai Electronic Commerce Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
1.Before use, clean the affected area with soap and soak the nail in warm water to soften it.
2.For toenail fungus, scrape the dirt off the nail and g ently file the thickened part of the fungal nail until
it's flat (be careful not to damage the nail bed).
3.You can use a nail file every 2-3 days to polish the surface of the affected area by applying a thin layer
of liquid to the affected area, the skin under the nail and around the cuticle.
4.Use twice daily, morning and evening.
5.Repeat this process 2-3 times a day until a healthy nail begins its gradual degeneration. For best results,
continue use for a continuous period of 6 weeks. - Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
FUNGAL NAIL TREATMENT
fungal nail treatment liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84291-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 mL CHLORHEXIDINE ACETATE (UNII: 5908ZUF22Y) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE ACETATE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ANGELICA DAHURICA LEAF (UNII: ONF5ZKM88G) LAUROCAPRAM (UNII: 1F3X9DRV9X) OXYMETHUREA (UNII: N68H97CAWG) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) CARAMEL (UNII: T9D99G2B1R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84291-003-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/04/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 09/04/2024 Labeler - Shenzhen Yuetai Electronic Commerce Co., Ltd (403120360) Establishment Name Address ID/FEI Business Operations Shenzhen Yuetai Electronic Commerce Co., Ltd 403120360 manufacture(84291-003)