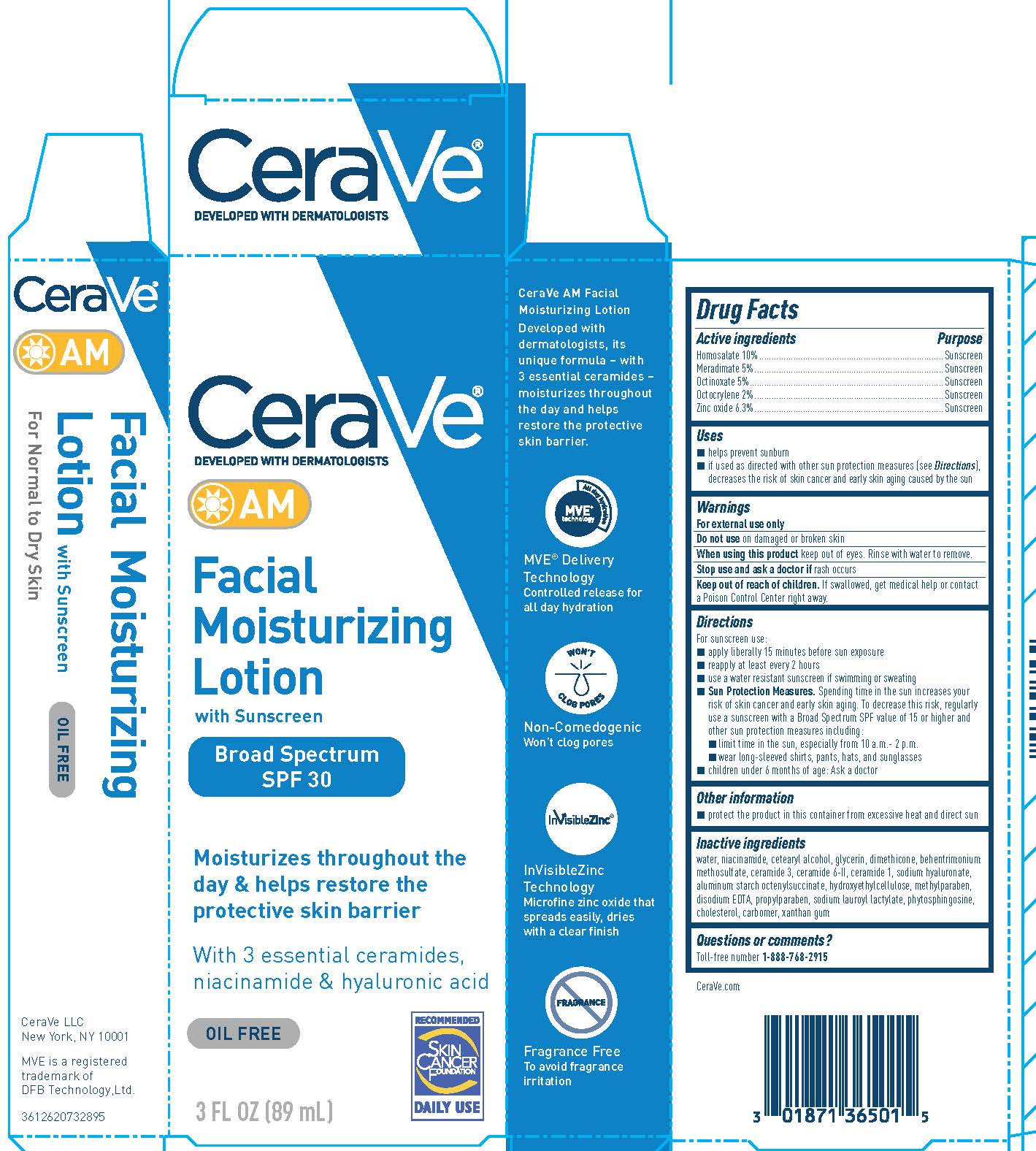
Label: CERAVE AM FACIAL MOISTURIZING BROAD SPECTRUM SPF 30 SUNSCREEN- homosalate, meradimate, octinoxate, zinc oxide, and octocrylene lotion
-
NDC Code(s):
49967-015-01,
49967-015-02,
49967-015-03,
49967-015-04, view more49967-015-05, 49967-015-06
- Packager: L'Oreal USA Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
For sunscreen use:
● apply liberally 15 minutes before sun exposure
● reapply at least every 2 hours
● use a water resistant sunscreen if swimming or sweating
● Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
● limit time in the sun, especially from 10 a.m. – 2 p.m.
● wear long-sleeved shirts, pants, hats, and sunglasses
● children under 6 months of age: Ask a doctor -
Inactive ingredients
water, niacinamide, cetearyl alcohol, glycerin, dimethicone, behentrimonium methosulfate, ceramide 3, ceramide 6-II, ceramide 1, hyaluronic acid, sodium hydroxide, aluminum starch octenylsuccinate, hydroxyethylcellulose, methylparaben, disodium EDTA, propylparaben, sodium lauroyl lactylate, phytosphingosine, cholesterol, carbomer, xanthan gum
- Other information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CERAVE AM FACIAL MOISTURIZING BROAD SPECTRUM SPF 30 SUNSCREEN
homosalate, meradimate, octinoxate, zinc oxide, and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 100 mg in 1 mL MERADIMATE (UNII: J9QGD60OUZ) (MERADIMATE - UNII:J9QGD60OUZ) MERADIMATE 50 mg in 1 mL Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 20 mg in 1 mL Zinc oxide (UNII: SOI2LOH54Z) (Zinc oxide - UNII:SOI2LOH54Z) Zinc oxide 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) NIACINAMIDE (UNII: 25X51I8RD4) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) CERAMIDE 1 (UNII: 5THT33P7X7) HYALURONIC ACID (UNII: S270N0TRQY) SODIUM HYDROXIDE (UNII: 55X04QC32I) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) HYDROXYETHYL CELLULOSE (4000 MPA.S AT 1%) (UNII: ZYD53NBL45) METHYLPARABEN (UNII: A2I8C7HI9T) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) CHOLESTEROL (UNII: 97C5T2UQ7J) CARBOMER HOMOPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: F68VH75CJC) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-015-01 1 in 1 CARTON 08/03/2017 1 89 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:49967-015-02 1 in 1 CARTON 08/03/2017 2 60 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:49967-015-03 5 mL in 1 TUBE; Type 0: Not a Combination Product 08/03/2017 4 NDC:49967-015-04 1.5 mL in 1 PACKET; Type 0: Not a Combination Product 08/03/2017 5 NDC:49967-015-05 1 in 1 CARTON 08/03/2017 5 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:49967-015-06 1 in 1 CARTON 08/03/2017 6 148 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/03/2017 Labeler - L'Oreal USA Products, Inc. (002136794) Establishment Name Address ID/FEI Business Operations L'Oreal USA Products, Inc. 624244349 MANUFACTURE(49967-015) , pack(49967-015) Establishment Name Address ID/FEI Business Operations Universal Packaging Systems, Inc. 078717086 pack(49967-015)