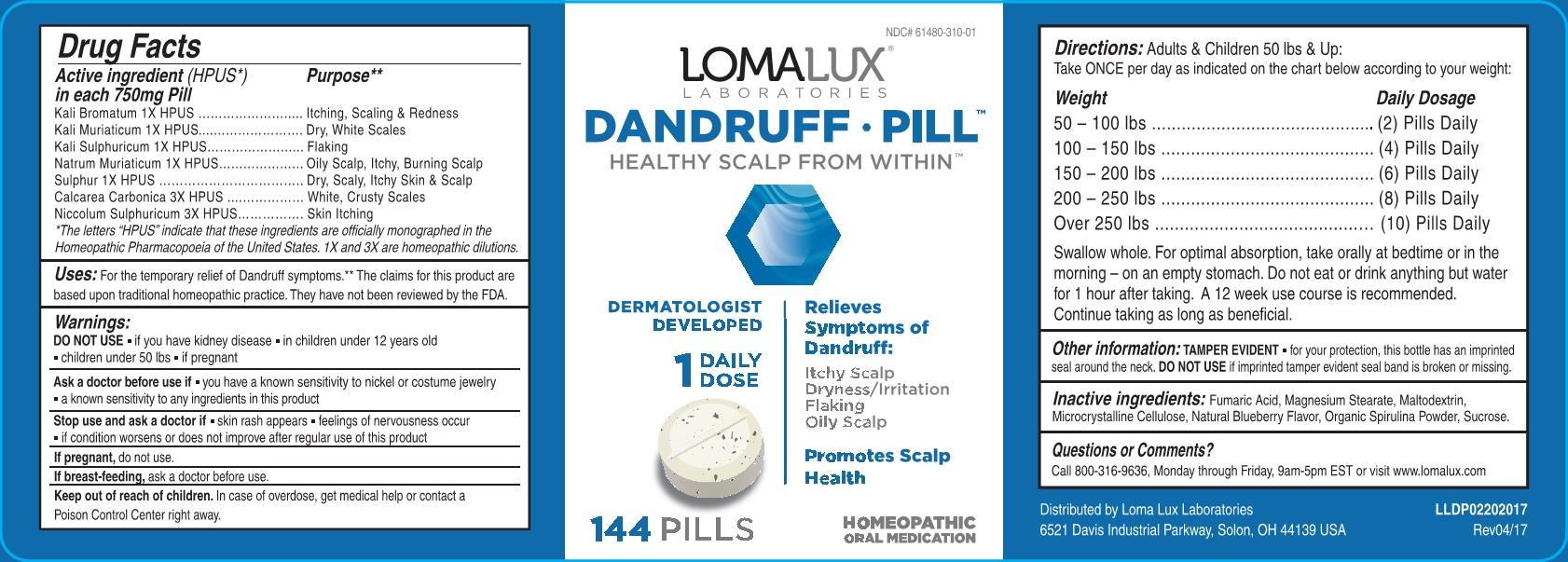
Label: DANDRUFF- kali bromatum, kali muriaticum, kali sulphuricum, natrum muriaticum, sulphur, calcarea carbonica, niccolum sulphuricum. tablet
- NDC Code(s): 61480-310-01
- Packager: PLYMOUTH HEALTHCARE PRODUCTS LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS / PURPOSE
Active ingredients (HPUS*) in each 750mg Pill Purpose** Kali Bromatum 1X HPUS Itching, Scaling & Redness Kali Muriaticum 1X HPUS Dry, White Scales Kali Sulphuricum 1X HPUS Flaking Natrum Muriaticum 1X HPUS Oily Scalp, Itchy, Burning Scalp Sulphur 1X HPUS Dry, Scaly, Itchy Skin & Scalp Calcarea Carbonica 3X HPUS White, Crusty Scales Niccolum Sulphuricum 3X HPUS Skin Itching - USES
-
WARNINGS
Warnings:
DO NOT USE
- if you have kidney disease
- in children under 12 years old
- children under 50 lbs
- if pregnant
Ask a doctor before use if
- you have a known sensitivity to nickel or costume jewelry
- a known sensitivity to any ingredients in this product
Stop use and ask a doctor if
- skin rash appears
- feelings of nervousness occur
- if condition worsens or does not improve after regular use of this product
If pregnant, do not use. If breast-feeding, ask a doctor before use. Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. -
DIRECTIONS
Directions: Adults & Children 50 lbs & Up:
Take ONCE per day as indicated on the chart below according to your weight:
Weight Daily Dosage
50 - 100 lbs ...................... (2) Pills Daily
100 - 150 lbs .................... (4) Pills Daily
150 - 200 lbs .................... (6) Pills Daily
200 - 250 lbs .................... (8) Pills Daily
Over 250 lbs ..................... (10) Pills Daily
Swallow whole. For optimal absorption, take orally at bedtime or in the morning - on an empty stomach. Do not eat or drink anything but water for 1 hour after taking.
- OTHER INFORMATION
- INACTIVE INGREDIENT
-
QUESTIONS or COMMENTS?
Questions or Comments?
Call 800-316-9636, Monday through Friday, 9am-5pm EST or visit www.lomalux.com
Distributed by Loma Lux Laboratories: 6521 Davis Industrial Parkway, Solon, OH 44139 USA
-
OTHER SAFETY INFORMATION
WE TARGET DANDRUFF AT ITS SOURCE - FOR HEALTHY SCALP FROM WITHIN
Dandruff Pill works from the inside out to provide scalp relief from within. Just like your daily vitamin, Dandruff Pill is taken orally. With just one daily dose, Dandruff Pill's active mineral ingredients help relieve Dandruff symptoms.
NATURE CREATED - DERMATOLOGIST PERFECTED
We use only the finest quality ingredients in our exclusive formulation developed by our dermatologist founder to promote scalp health from within. A perfect blend of science and nature.
MADE IN THE USA
NATURAL active ingredients
LACTOSE FREE
VEGAN
GLUTEN FREE
EXCLUSIVE MINERAL TECHNOLOGY PROMOTES SCALP HEALTH
7 Active Mineral Ingredients help relieve dandruff symptoms of flaking, itchy scalp, dryness, and irritation. These unique inorganic minerals are all natural, earth derived.
WHEN USED REGULARLY, MAY HELP RELIEVE SYMPTOMS OF DANDRUFFDERMATOLOGISTS DEVELOPED, FOR HEALTHY SKIN FROM WITHIN
UNIQUE! - CUSTOMED DOSAGE ACCORDING TO YOUR WEIGHT FOR OPTIMAL RESULTS
A 12 WEEK USE COURSE IS RECOMMENDED. CONTINUE TAKING AS LONG AS BENEFICIAL.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DANDRUFF
kali bromatum, kali muriaticum, kali sulphuricum, natrum muriaticum, sulphur, calcarea carbonica, niccolum sulphuricum. tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61480-310 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM BROMIDE (UNII: OSD78555ZM) (BROMIDE ION - UNII:952902IX06) POTASSIUM BROMIDE 1 [hp_X] POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CATION 1 [hp_X] POTASSIUM SULFATE (UNII: 1K573LC5TV) (SULFATE ION - UNII:7IS9N8KPMG) POTASSIUM SULFATE 1 [hp_X] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 1 [hp_X] SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 1 [hp_X] OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 3 [hp_X] NICKEL SULFATE HEXAHYDRATE (UNII: JC9WZ4FK68) (NICKEL CATION - UNII:OIS2CXW7AM) NICKEL SULFATE HEXAHYDRATE 3 [hp_X] Inactive Ingredients Ingredient Name Strength FUMARIC ACID (UNII: 88XHZ13131) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) BLUEBERRY (UNII: 253RUG1X1A) SPIRULINA PLATENSIS (UNII: 9L3TIH1UUE) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (off-white with speckles) Score 2 pieces Shape ROUND Size 11mm Flavor BLUEBERRY Imprint Code LL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61480-310-01 144 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/24/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/24/2017 Labeler - PLYMOUTH HEALTHCARE PRODUCTS LLC (079330314)