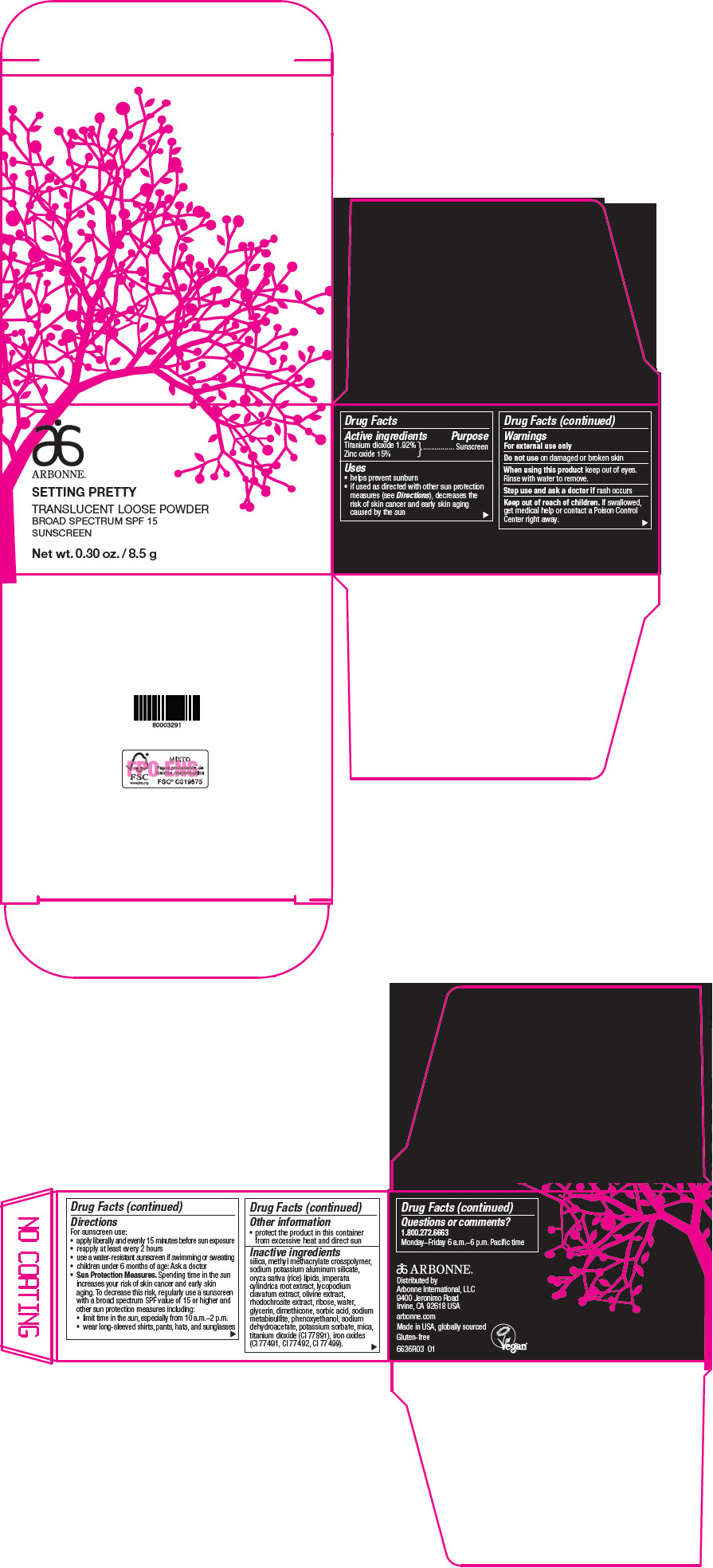
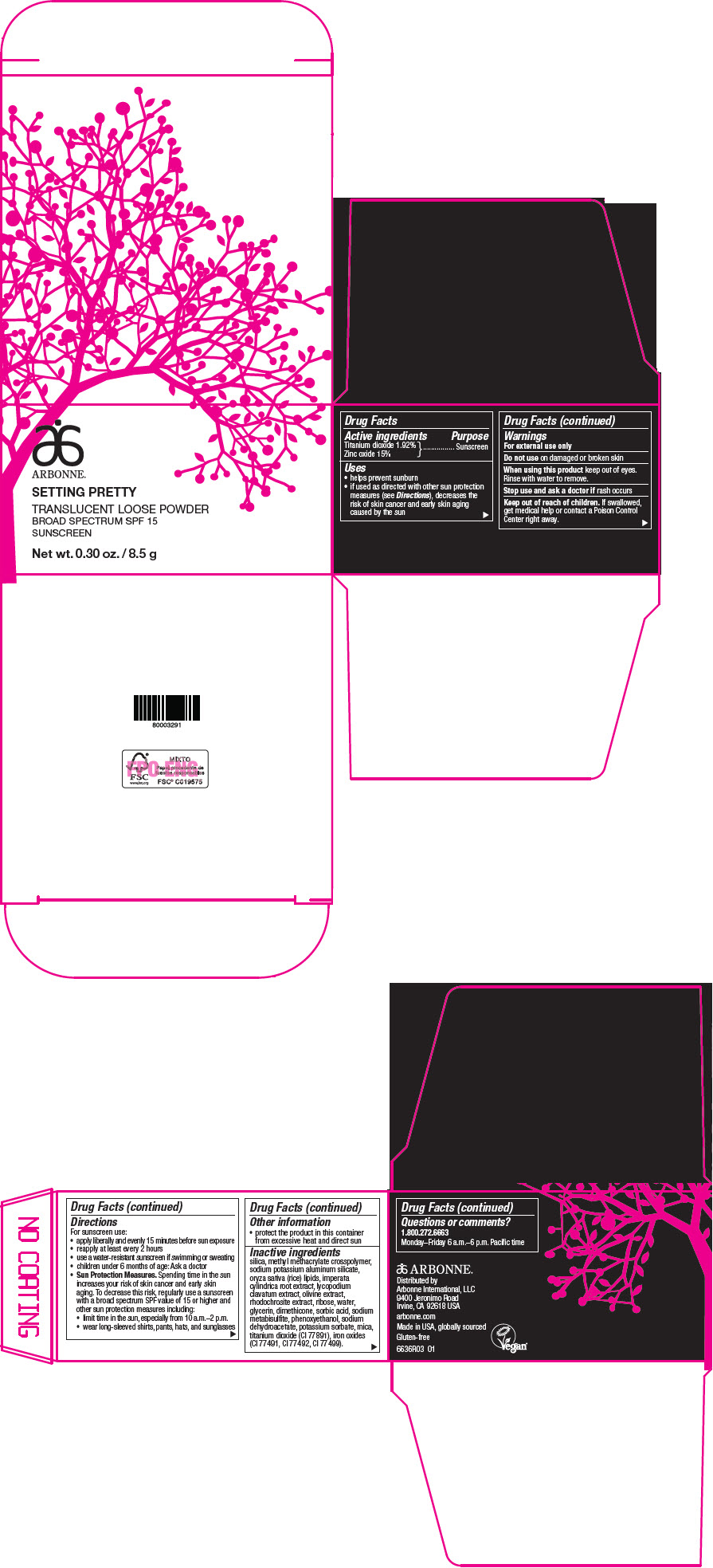
Label: ARBONNE SETTING PRETTY TRANSLUCENT BROAD SPECTRUM SPF 15 SUNSCREEN- titanium dioxide and zinc oxide powder
- NDC Code(s): 42508-013-01
- Packager: Arbonne International, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
- children under 6 months of age: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Other information
-
Inactive ingredients
silica, methyl methacrylate crosspolymer, sodium potassium aluminum silicate, oryza sativa (rice) lipids, imperata cylindrica root extract, lycopodium clavatum extract, olivine extract, rhodochrosite extract, ribose, water, glycerin, dimethicone, sorbic acid, sodium metabisulfite, phenoxyethanol, sodium dehydroacetate, potassium sorbate, mica, titanium dioxide (CI 77891), iron oxides (CI 77491, CI 77492, CI 77499).
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 8.5 g Jar Carton
-
INGREDIENTS AND APPEARANCE
ARBONNE SETTING PRETTY TRANSLUCENT BROAD SPECTRUM SPF 15 SUNSCREEN
titanium dioxide and zinc oxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42508-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 19.2 mg in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 150 mg in 1 g Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) IMPERATA CYLINDRICA ROOT (UNII: VYT2JA85NH) LYCOPODIUM CLAVATUM WHOLE (UNII: 005ICF6L27) OLIVINE (UNII: 95548S7QBV) MANGANESE CARBONATE (UNII: 9ZV57512ZM) RIBOSE, D- (UNII: 681HV46001) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) SORBIC ACID (UNII: X045WJ989B) SODIUM METABISULFITE (UNII: 4VON5FNS3C) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42508-013-01 1 in 1 CARTON 05/01/2020 08/01/2024 1 8.5 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 05/01/2020 08/01/2024 Labeler - Arbonne International, LLC (961643454)