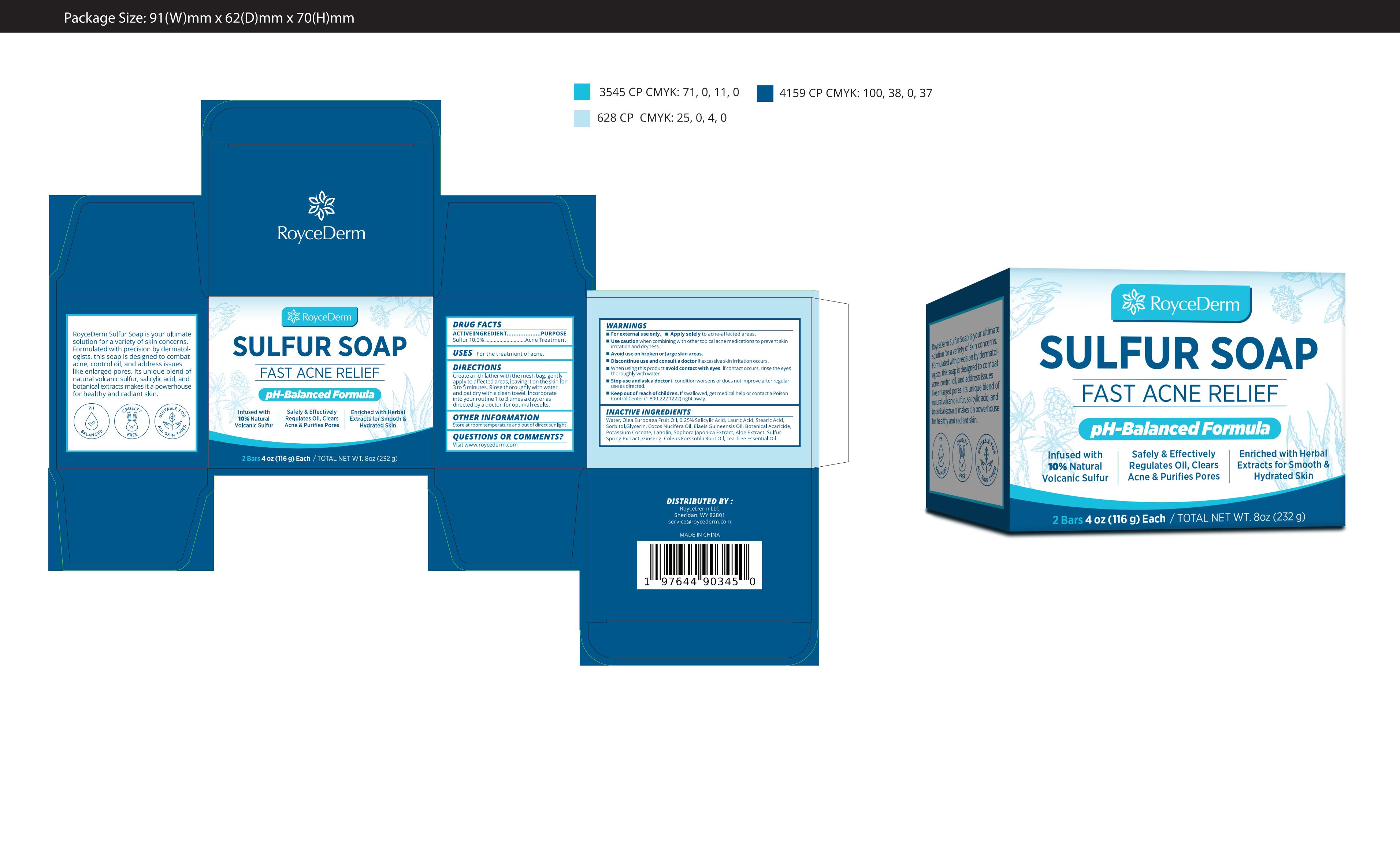
Label: ROYCEDERM SULFUR SOAP.- sulfur soap soap
- NDC Code(s): 82739-023-01
- Packager: Shenzhen Situya Trading Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Other information
-
Inactive ingredients
Water, Olea Europaea Fruit Oil, 0.25% Salicylic Acid, Lauric Acid, Stearic Acid, Sorbitol,Glycerin, Cocos Nucifera Oil, Elaeis Guineensis Oil, Botanical Acaricide, Potassium Cocoate, Lanolin, Sophora Japonica Extract, Aloe Extract, Sulfur Spring Extract, Ginseng, Coleus Forskohlii Root Oil, Tea Tree Essential Oil.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ROYCEDERM SULFUR SOAP.
sulfur soap soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82739-023 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 10 g in 100 g Inactive Ingredients Ingredient Name Strength SALICYLIC ACID (UNII: O414PZ4LPZ) SORBITOL (UNII: 506T60A25R) COCONUT OIL (UNII: Q9L0O73W7L) LANOLIN (UNII: 7EV65EAW6H) ALOE (UNII: V5VD430YW9) TEA TREE OIL (UNII: VIF565UC2G) WATER (UNII: 059QF0KO0R) POTASSIUM COCOATE (UNII: F8U72V8ZXP) PALM OIL (UNII: 5QUO05548Z) CAMPHECHLOR (UNII: 9924JQ4D5J) STYPHNOLOBIUM JAPONICUM ROOT (UNII: LAH7966318) 4-METHYL-5-THIAZOLEETHANOL (UNII: 3XYV4I47I8) OLIVE OIL (UNII: 6UYK2W1W1E) STEARIC ACID (UNII: 4ELV7Z65AP) LAURIC ACID (UNII: 1160N9NU9U) GLYCERIN (UNII: PDC6A3C0OX) ASIAN GINSENG (UNII: CUQ3A77YXI) COLEUS FORSKOHLII ROOT OIL (UNII: 5NXR7DJ9WO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82739-023-01 232 g in 1 BOX; Type 0: Not a Combination Product 09/02/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 09/02/2024 Labeler - Shenzhen Situya Trading Co., Ltd. (706154255) Establishment Name Address ID/FEI Business Operations Shenzhen Situya Trading Co., Ltd. 706154255 manufacture(82739-023) , label(82739-023)