Nicotine Polacrilex Lozenge
-
2 mg and 4 mg User’s Guide
-
How to Use Nicotine Polacrilex Lozenges and Tips to Help You Quit Smoking.
PLANNING YOUR SUCCESS
-
1)
The key to accomplishing ...
Nicotine Polacrilex Lozenge
2 mg and 4 mg User’s Guide
How to Use Nicotine Polacrilex Lozenges and Tips to Help You Quit Smoking.
PLANNING YOUR SUCCESS
- 1)
- The key to accomplishing anything important is commitment. When it comes to quitting smoking, that is especially true. Nicotine Polacrilex Lozenges can help if you really want to quit. Nicotine Polacrilex Lozenges help reduce withdrawal symptoms including nicotine craving associated with quitting smoking.
- 2)
- Your chances of staying off cigarettes are much better if you start with at least 9 Nicotine Polacrilex Lozenges daily. For best results, use the lozenges on a regular schedule (as outlined in this User’s Guide).
- 3)
- Start using Nicotine Polacrilex Lozenges on your quit date.
- 4)
- This User’s Guide outlines a 12-week plan for Nicotine Polacrilex Lozenges. Even though you may feel confident about your non-smoking status after a few weeks, it’s important to stick with the plan to help you remain smoke free. Even a single cigarette can put you right back to square one.
- 5)
- Nicotine Polacrilex Lozenges work best when used together with a support plan. See information to the right for instructions on enrollment in the SmokeFreeHabits.com Free & Personalized Support Plan.
- 6)
- After the first six weeks, start using fewer Nicotine Polacrilex Lozenges, as directed in the instructions, gradually reducing your use over the next six weeks. If you feel the need to use the lozenges for a longer period to keep from smoking, talk to your health care provider.
- 7)
- If you have questions about using Nicotine Polacrilex Lozenges, call toll-free 1-866-751-9303, or talk to your pharmacist or family doctor.
YES! YOU WANT TO QUIT.
Wonderful. You’ve made the most important decision of all, to stop smoking. And by choosing Nicotine Polacrilex Lozenges to help you, you’re starting on the right path. Now remember, using Nicotine Polacrilex Lozenges doesn’t just mean taking a Nicotine Polacrilex Lozenge. It means setting and following a program like the one we suggest in this User’s Guide.
Your own success depends on your effort, your level of addiction to tobacco, and your commitment to following your program.
LET’S FACE IT.
Quitting smoking isn’t easy! You or someone you know may have tried unsuccessfully. That’s okay. It’s hard to stop smoking the first time you try. The important part is to learn from your previous attempts, consider what went wrong and keep trying to quit until you succeed. Look to this User’s Guide for support as you undergo this terrific task. The guide includes important information on how to use Nicotine Polacrilex Lozenges and also gives you tips to help you stop smoking. Refer back to it often for advice, answers, and encouragement to help you stay on track.
GET MOTIVATED. STAY MOTIVATED.
Everyone has a reason for quitting–whether you’re concerned about your health, your appearance, family or peer pressure, or the effect of secondhand smoke on your loved ones–all of the above, or something else entirely. Whatever your reasons, write them down. There’s a wallet card on the bottom of this User’s Guide. Write your reasons on the card and carry it with you. When you have an urge to smoke or experience a difficult moment it can help you focus on your reasons for quitting. Lots of people quit with a co-worker, spouse or friend and use them as a quitting buddy. You can help each other out by providing extra encouragement in tough moments.
There may be support groups in your area for people trying to quit. Call your local chapter of the American Lung Association, American Cancer Society or American Heart Association for further information. Toll-free phone numbers are printed on the wallet card on the bottom of this User’s Guide.
UNDERSTANDING THE DOUBLE-EDGED SWORD.
Smoking has two addictive components, a physical and a mental need for the nicotine in tobacco. You need to conquer both to succeed. Nicotine Polacrilex Lozenges can ease your physical nicotine addiction. But your readiness and resolve are necessary to help overcome the mental side of your cigarette dependence. So once you’re ready, it’s time to begin. But first, read and consider the following important warnings.
IMPORTANT WARNINGS
This product is only for those who want to stop smoking. If you are pregnant or breast-feeding, only use this medicine on the advice of your health care provider. Smoking can seriously harm your child. Try to stop smoking without using any nicotine replacement medicine. This medicine is believed to be safer than smoking. However, the risks to your child from this medicine are not fully known.
Ask a doctor before use if you have
- •
- heart disease, recent heart attack or irregular heartbeat. Nicotine can increase your heart rate.
- •
- high blood pressure not controlled with medication. Nicotine can increase your blood pressure.
- •
- stomach ulcers or diabetes.
- •
- history of seizures
Ask a doctor or pharmacist before use if you are
- •
- using a non-nicotine stop smoking drug
- •
- taking prescription medicine for depression or asthma. Your prescription dose may need to be adjusted.
Stop use and ask a doctor if
- •
- mouth problems occur
- •
- persistent indigestion or severe sore throat occurs
- •
- irregular heartbeat or palpitations occur
- •
- you get symptoms of nicotine overdose such as nausea, vomiting, dizziness, diarrhea, weakness and rapid heartbeat
- •
- you have symptoms of an allergic reaction (such as difficulty breathing or rash)
Keep out of reach of children and pets. Nicotine lozenges may have enough nicotine to make children and pets sick. If you need to remove the lozenge, wrap it in paper and throw away in the trash. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
YOU’RE READY TO START.
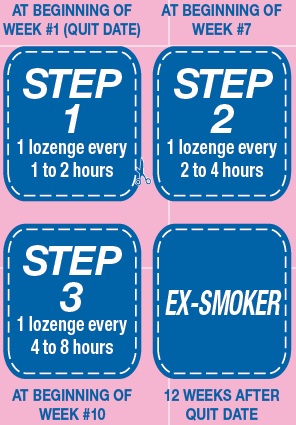
Okay, you’re ready. To become a non-smoker, start today. Now before you do anything else, you have a bit of planning to do. Read this User’s Guide all the way through. You want to make sure you bought the right dose to start. If you typically smoke your first cigarette within 30 minutes of waking up, use the 4 mg Nicotine Polacrilex Lozenges. If you smoke your first cigarette more than 30 minutes after waking up, use the 2 mg Nicotine Polacrilex Lozenges. Next, plan your quitting schedule. Get a calendar to follow your progress and mark the following four important dates (see the reminders on the left side of this leaflet).
THE PROGRAM
STEP 1. (Weeks 1-6) Starting on your quit date it’s best to use at least 9 Nicotine Polacrilex Lozenges each day, one every 1-2 hours.
First choose the day you plan to quit (make it soon). Place the Step 1 reminder on this date. That’s the day you will start using Nicotine Polacrilex Lozenges to calm your cravings for nicotine and help you stay smoke free. Prior to the quit date, get rid of all your cigarettes to remove temptations and make it more difficult to start smoking again.
Use a Nicotine Polacrilex Lozenge every 1 to 2 hours and at least 9 lozenges each day for the first 6 weeks to help prevent unexpected cravings and improve your chances of quitting. These aren’t ordinary lozenges. Place the lozenge in your mouth and allow the lozenge to slowly dissolve. Minimize swallowing. Do not chew or swallow the lozenge. You may feel a warm or tingling sensation. Occasionally move the lozenge from one side of your mouth to the other until completely dissolved. Remember to read the “USING Nicotine Polacrilex LOZENGES PROPERLY” section before you take your first Nicotine Polacrilex Lozenge.
STEP 2. (The next three weeks, that is weeks 7-9). At the beginning of week 7 start using fewer Nicotine Polacrilex Lozenges, one every 2-4 hours.
After six weeks, you should wait a little longer between lozenges, one lozenge every two to four hours. This will help you gradually use fewer Nicotine Polacrilex Lozenges. Put the Step 2 reminder on the first day of week 7 to help remind you when to start reducing the number of Nicotine Polacrilex Lozenges you take.
STEP 3. (The last three weeks, that is weeks 10-12). At the beginning of week 10, reduce Nicotine Polacrilex Lozenge use even further, one every 4-8 hours.
At the beginning of week 10 further decrease the number of Nicotine Polacrilex Lozenges you use each day to reduce the amount of nicotine you get. You should do this by using one lozenge every 4 to 8 hours. Put the Step 3 reminder on the first day of week 10 so you know when you should be starting this last step to becoming smoke and nicotine-free.
END. At the end of week 12 you’ll complete Nicotine Polacrilex Lozenge therapy.
Put the “EX-SMOKER” reminder on your calendar on the date 12 weeks after the day you stopped smoking and started using Nicotine Polacrilex Lozenges.
BE PREPARED.
Since smoking is an addiction, it is hard to quit. Even after you stop, there will be times when you WANT a cigarette, sometimes strongly (see also section on “CHALLENGES TO WATCH FOR”). The best defense is to be prepared. Plan now for handling tough times so you don’t give in. Think about situations where you usually get an urge to smoke or where you think you might experience strong urges to smoke (for example, spending time with smokers or drinking alcohol – try to avoid these situations if those things tempt you to smoke).
Change your habits. Take your coffee break somewhere else. Take a walk. Break the association between your usual habits and cigarettes.
If you do encounter a situation where you feel a strong urge to smoke, fight it! Take a break from the situation; keep yourself busy or distracted with other activities. Remind yourself why you want to quit, and that having “just one” will really hurt your goal of quitting!
Assemble a “survival package” – items that can keep you distracted in case you get an urge to smoke. For example, cinnamon gum or hard candy, relaxing music, things to keep your hands busy like a smooth stone, or paperclips, etc.
Track your quit progress. Use a journal to note if and when you get an urge to smoke. Note how many pieces of Nicotine Polacrilex Lozenges you use each day. If you slip and have a cigarette, don’t give up. Stop smoking again and get back on your program with Nicotine Polacrilex Lozenges.
Establish your support network. Keep friends’ and family members’ phone numbers ready to get the moral support you need. Before quitting, ask friends and family to support and encourage you. Think of specific ways they can help.
Reward yourself. Set aside little gifts to encourage yourself which you can earn by overcoming difficult hurdles.
HOW Nicotine Polacrilex LOZENGES WORK.
Nicotine Polacrilex Lozenges are a form of Nicotine Replacement Therapy. They deliver nicotine to your body, temporarily relieving craving and nicotine withdrawal symptoms when you quit smoking. But unlike cigarettes, Nicotine Polacrilex Lozenges deliver a lower, steady level of nicotine to your blood. When used as directed, Nicotine Polacrilex Lozenges help you regulate, control, and gradually reduce your body’s craving for nicotine.
The good news is that Nicotine Polacrilex Lozenges contain no tar or carbon monoxide, and therefore don’t present the same medical risks as cigarettes.
However, the lozenges still deliver nicotine, the addictive ingredient in cigarettes. And for some people the nicotine in Nicotine Polacrilex Lozenges can occasionally cause mouth or throat irritation, headaches, nausea, hiccups, upset stomach or dizziness.
USING Nicotine Polacrilex LOZENGES PROPERLY.
Remember, Nicotine Polacrilex Lozenges aren’t like ordinary lozenges such as cough drops. This lozenge is designed to deliver nicotine into your system through the lining of your mouth, not in your stomach like most other medicines. It is important to minimize swallowing the dissolved medicine in these lozenges so that it can be properly absorbed in your mouth.
Do not use more than one lozenge at a time, or many lozenges one after another since this can cause hiccups, heartburn, nausea or other side effects.
Read all the following instructions before using Nicotine Polacrilex Lozenges. Refer to them often to make sure you’re using Nicotine Polacrilex Lozenges correctly.
IMPORTANT: Don’t worry or give up if you do not like the taste of the lozenge at first. Nicotine Polacrilex Lozenges are a medication, not a candy. Most people get used to the taste after a day or two. Remember, staying with the plan will help you quit. Begin using Nicotine Polacrilex Lozenges on your quit date.
- 1)
- Remove the Nicotine Polacrilex Lozenge from the immediate container. Place the lozenge in your mouth and allow the lozenge to slowly dissolve. Minimize swallowing.
-
-
Do not chew or swallow the lozenge. You may feel a warm or tingling sensation.
- 2)
- Occasionally move the lozenge from one side of your mouth to the other side until completely dissolved.
To reduce cravings or urges to smoke and other withdrawal symptoms, use Nicotine Polacrilex Lozenges according to the following dosage schedule:
Do not use more than 5 lozenges in 6 hours. Do not use more than 20 lozenges per day. At the end of 12 weeks (3 months) you will have completed treatment.
FOR THE BEST CHANCE OF QUITTING, use Nicotine Polacrilex Lozenges on a regular schedule, using at least 9 lozenges a day during the first 6 weeks. That will help your body better adjust to the lack of cigarettes and better help prevent cravings. Some people may need more lozenges to reduce their cravings. Do not exceed the recommended maximum daily dosage of 20 lozenges per day. Do not continuously use one lozenge after another, since this may cause you hiccups, heartburn, nausea or other side effects.
Do not eat or drink 15 minutes before using or while the lozenge is in your mouth.
CUTTING BACK ON YOUR Nicotine Polacrilex LOZENGE USAGE.
The whole reason for using Nicotine Polacrilex Lozenges is to decrease and slowly eliminate your need for nicotine, while you control cravings. So, as the above schedule indicates, you should gradually reduce the amount of Nicotine Polacrilex Lozenges you take per day. Some people find it easier to reduce by substituting ordinary sweets or sugar free candy for some of the Nicotine Polacrilex Lozenges they would normally use. As time goes on, you can increase the number of pieces of candy as you further reduce your use of Nicotine Polacrilex Lozenges. It is important to complete treatment. If you still feel the need to use Nicotine Polacrilex Lozenges to keep from smoking after week 12, talk with your health care provider.
MAKE QUITTING EASIER ON YOURSELF.
Soon after your quit date, parties, bars, celebrations, and socializing may all tempt you to smoke. Please remember these tips to help you resist those urges and stay smoke-free.
The Day You Quit Smoking:
- •
- Look to your family and friends for support. Let them know what to do or avoid doing to help you quit.
- •
- Throw away ALL cigarettes, ashtrays, matches, lighters. You don’t need them. You don’t want them and you want to make it difficult to go back.
- •
- Keep yourself occupied. Take a walk. See a movie. See friends. Do anything to keep your mind off cigarettes.
- •
- Calculate all the money you’ll save by not buying cigarettes. Probably well over $1,000 a year! $1,000 a year? Think of what you can spend it on!
- •
- Know what situations are going to make you want to smoke. Plan now how you’ll avoid them or deal with them so you don’t smoke.
- •
- Keep Nicotine Polacrilex Lozenges next to your bed so you’re prepared when you get up. A lot of people get cravings first thing in the morning.
- •
- Make an appointment to see your dentist and get the tobacco stains cleaned off. While you’re getting rid of the evidence of cigarettes in the house, do the same for your teeth. Have clothes or drapes that smell of smoking cleaned.
- •
- Now that your house is smoke-free, try to spend most of your time in smoke-free environments.
- •
- If you usually smoked with coffee or alcohol, try to keep away from them for now. Remember you are also trying to break a habit.
- •
- Smoking is a “hands-on” habit. So use something else to occupy your hands: a rubber band or a pen.
- •
- Now’s a good time to get active. Find activities to take your mind off cigarettes and relax. Take up jogging, swimming, or walking.
- •
- Don’t stress out about gaining weight. Dieting now may weaken your efforts to quit smoking. Eat sensibly and exercise daily; drink large quantities of water and fruit juices; this can help your chances of staying smoke-free.
- •
- Laugh. Watch a sitcom. Read a comic book. It really helps.
REMEMBER: Urges to smoke are temporary. They’ll pass, even if you don’t smoke.
WHAT YOU CAN EXPECT.
As you are successful at staying smoke-free, initially you will probably notice a few of the following typical withdrawal symptoms, so don’t be surprised. Use of Nicotine Polacrilex Lozenges reduces these symptoms, but may not eliminate them entirely. They will go away with time. Stay focused on your goal of becoming an ex-smoker. Research shows that if you manage to avoid all smoking in the first week (that means not having a single puff), your chances of success increase dramatically.
The First Few Days. You may feel nervous or irritable or have difficulty concentrating during the first few days after you quit smoking. Your body needs time to regain balance. Initially, you might feel a little out of sorts, get headaches, feel light-headed, or have trouble sleeping. Your smoker’s cough may get worse before it improves. But fear not, it’s a positive sign. Coughing helps clean your lungs of the tar residue you got from smoking.
After a Couple of Weeks. Your confidence and ability to cope with urges to smoke should be getting stronger. But don’t be over-confident and think you can smoke just one cigarette. Even now, having even a single puff can lead to a return to smoking cigarettes regularly. Be prepared, and remember why you wanted to stop smoking.
Have you noticed that your sense of taste and smell has improved? You are probably coughing less and finding it easier to breathe. You’ve also probably noticed your withdrawal symptoms are subsiding (though don’t worry if they’re still there: they last longer for some people). These are all positive signs that your body is getting used to your success at stopping smoking.
By The End of The First Month. You are less likely to have cravings for cigarettes as often. However sudden cravings may still happen, and when they do, be on your guard, as they can be strong and seem to come out of the blue. Be prepared for these challenging times. The key is do what you can so these unexpected cravings can’t beat you. Keep focused on the ways non-smokers are more attractive than smokers. Their breath smells better. Their clothes and hair are fresher. Their teeth are cleaner and brighter. Their skin is less likely to wrinkle. Not smoking around children and your friends is also healthier for them too.
What If You Do Slip And Smoke?
“What if I relapse?” One cigarette is a slip-up, but it’s not the end of the quit effort. Everybody slips at something. The key is this: forgive yourself and stop at that one cigarette. Don’t let this slip ruin your good intentions, keep at your quit attempt. So, throw out your cigarettes and continue with your quit attempt, keeping in mind what went wrong and led to the slip.
If you do go back to smoking, certainly don’t throw out your Nicotine Polacrilex Lozenges. Keep them for the next time you’re ready to quit. In fact research says that even if you are back to smoking regularly the best thing you can do is learn and try again.
Try to understand the reason you had those cigarettes that made you slip. That’s important, because now you can plan better to deal with these moments next time. It’s true you stumbled, but don’t think of yourself as having failed. Encourage yourself by treating the last attempt as a learning experience, even a “trial run” for the real thing.
Take a look at the usage instructions and check that you used the Nicotine Polacrilex Lozenges correctly and for the full 12 weeks of the program. When you try again make sure you use enough and the right way. That way you’ll be best equipped to deal with the unexpected cravings.
Don’t forget; quitting isn’t easy and it takes practice to do anything. Stopping smoking is no different.
YOU’VE MADE IT.
Once your twelve week quitting program is over, you’ve taken your last Nicotine Polacrilex Lozenge. Now you are both cigarette and nicotine-free. Get up and give yourself a standing ovation. We mean it. Do you realize that you have just done a really difficult thing?
Now’s a good time to think back on the process. Think of all your reasons for quitting smoking. Think of your goals. Think of how they’re going to be a reality now.
Think of what you’re going to do with your newly liberated cigarette money. The places you can now go smoke-free. Think of the extra time you may have added to your life and what you can do with it. And although you may still experience the occasional temptation, and cigarettes still want you back, think positively. Think forward. And consider yourself a proud non-smoker.
FREQUENTLY ASKED QUESTIONS.
1. When I stop smoking and start using Nicotine Polacrilex Lozenges how will I feel?
Nicotine Polacrilex Lozenges help reduce cravings, but be prepared for some nicotine withdrawal symptoms. After you stop smoking they can begin almost at once and are normally at their strongest during the first three or four days. For some people, any of the following may occur:
• unexpected craving or urges for cigarettes
• anxiety, irritability, restlessness, mood changes, nervousness
• drowsiness
• trouble concentrating
• increased appetite and weight gain
• headaches, muscular pain, constipation, fatigue
Nicotine Polacrilex Lozenges are designed to reduce the craving for nicotine you used to satisfy with cigarettes. Nicotine Polacrilex Lozenges can also help provide relief from other withdrawal symptoms such as irritability and nervousness.
2. Are Nicotine Polacrilex Lozenges just swapping one type of nicotine addiction for another?
Nicotine Polacrilex Lozenges do contain nicotine, however there is probably less nicotine in your daily dose of lozenges than in your cigarettes. Nicotine Polacrilex Lozenges give you enough nicotine to help you combat the physical withdrawal symptoms so you can cope with the mental side of stopping smoking. Also, since the nicotine from the lozenges goes into your bloodstream more slowly, it produces less of the effects of nicotine that people find rewarding. In fact, when used as directed in the 12 week program, Nicotine Polacrilex Lozenges gradually wean you off your dependence for both nicotine and cigarettes.
3. Can Nicotine Polacrilex Lozenges do any harm?
Some people with conditions like heart disease or people taking prescription medicine for asthma or depression should not use this product without talking to their doctor–check the IMPORTANTWARNINGS” on the front of this leaflet. You may also experience side effects such as hiccups, mouth or throat irritation, heartburn or other stomach problems such as nausea especially if Nicotine Polacrilex Lozenges are chewed or swallowed. In any case, Nicotine Polacrilex Lozenges do not contain the tar, carbon monoxide, and other toxins present in cigarette smoke.
4. Will I put on weight?
In the first couple of months after quitting smoking, some people do put on a few pounds. But think of it this way. Overall, you’ll be healthier and look better. You can always tackle your weight by changing your diet and increasing the amount you exercise once you have gotten through the difficult part of stopping smoking.
5. Does taking Nicotine Polacrilex Lozenges cost more than smoking?
If you normally smoke a pack and a half a day, your total cost of using Nicotine Polacrilex Lozenges during the 12-week period should be less than smoking. But guess what? After you’ve finished the Nicotine Polacrilex Lozenge program all that money you used to spend on cigarettes is now savings. And think of the health issues you’ll hopefully be able to avoid.
6. What if I have a cigarette and start smoking?
Don’t panic. First, don’t think badly of yourself. Throw away your cigarettes and forgive yourself. Then think about what went wrong and get back on track. In fact people who have already tried to stop smoking are more likely to be successful the next time.
CHALLENGES TO WATCH FOR.
Once you quit smoking, you are likely to experience periodic, and sometimes intense, temptations to smoke. Certain situations present special challenges. Some common ones include:
Stress and upset.
When you are feeling stressed or upset, you may think a cigarette will make everything better. It won’t. Find other ways to relax and unwind.
The blues.
You may be especially vulnerable when you feel bored or blue. Remember that having a cigarette will just make you feel worse.
Smoking cues.
Seeing cigarettes or watching other people smoke can trigger temptation. Remember that you choose not to smoke anymore.
Alcohol.
Drinking and smoking seem to go together, and alcoholic beverages may weaken your resolve, making drinking dangerous to your quit effort. Avoid drinking early in your quit effort, and try to drink with non-smokers.
Automatic slips.
Sometimes you may find yourself preparing to smoke without even realizing it. Watch out for those moments when your hand seems to ‘automatically’ reach for a cigarette.
Watch out for these situations: they can trigger a relapse. You probably know which one(s) are most dangerous for you; plan ahead to deal with the situation effectively. Always remember that you’re trying to break a habit, and the most important thing is to do something to combat the urge in these situations.
COPING AFTER QUITTING.
The key to staying smoke-free is to prepare for and cope with challenges as they occur. If you find yourself tempted to smoke, do something! Here are some things to consider.
- •
- Escape. Leave the situation, even for a few minutes. Most temptations don’t last long.
- •
- Distract yourself. Get your mind off smoking. Think of something else or get busy with something.
- •
- Relax. Don’t let stress get to you. Think of pleasant, relaxing things; breathe slowly and regularly. Let the stress drain out of you.
- •
- Talk yourself out of it. What you say to yourself matters. So, remind yourself how important it is for you to quit; remind yourself you can’t have just one; or just command yourself to STOP.
For more information please visit www.SmokeFreeHabits.com
Packaged By
Perrigo®
Allegan, MI 49010
Rev. 08-22
WALLET CARD

WALLET CARD
: 74M0000J3
Free Personalized Plan
Begin Right Now!
Having a plan will help you in your efforts to stop smoking. We can help! Follow the simple steps below to receive your valuable personalized Smoke Free Habits plan and many other stop smoking tools and resources.
How To Enroll
Stopping smoking involves breaking your physical addiction and changing your behavior. You will use Nicotine Polacrilex Lozenges to help break your physical addiction, while your free personalized plan will help you develop healthier behaviors.
To enroll:
- •
- Go online to www.SmokeFreeHabits.com
- •
- Enter the first five digits of the UPC number from the box.
- •
- Answer the questions about yourself.
- •
- Print your personalized plan.
(If you don't have access to the Internet, you can call 1-866-751-9303, answer questions, and your plan will be mailed to you in a few days.)
What You Will Receive
Here are the free tools and resources that you will receive based on how you sign up for the program:
Tips To Get Started
- 1.
- Follow your personalized Smoke Free Habits support plan.
- 2.
- Be sure to use Nicotine Polacrilex Lozenges as directed.
- 3.
- Throw away all of your cigarettes, lighters, and ashtrays.
- 4.
- You may feel urges to smoke, but they usually pass in 2-3 minutes. When you feel an urge, do something else. Take deep breaths and let them out slowly. Drink a glass of water.
- 5.
- Carry things to put in your mouth, like gum, hard candy or toothpicks.
- 6.
- Be active. Take a walk with a friend, ride your bike, walk the dog, play tennis.
- 7.
- Go to places where you are not allowed to smoke, like the movies or the mall. Try to steer clear of places where you usually smoked, like a break room at work or a favorite bar.
- 8.
- Ask friends or family for support whenever you need it.
PLACE THESE REMINDERS ON YOUR CALENDAR:
Close