Label: CVS HEALTH ECZEMA SKIN RELIEF SKIN PROTECTANT- oatmeal lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 69842-962-01 - Packager: CVS Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 29, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
water, glycerin, glyceryl stearate, cetearyl alcohol, ceteareth-20, propanediol, dimethicone, cetyl alcohol, hydrolyzed jojoba esters, petrolatum, tetrasodiun EDTA, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, isohexadecane, polysorbate 60, aloe barbadensis leaf juice, avena sativa (oat) kernel extract, butyrospermum parkii (shea) butter extract, zingiber officinale (ginger) root extract, C12-15 alkyl benzoate, panthenol, ceramide NP, tocopheryl acetate, magnesium ascorbyl phosphate, bisabolol, citric acid, benzyl alcohol, ethylhexylglycerin.
- SPL UNCLASSIFIED SECTION
-
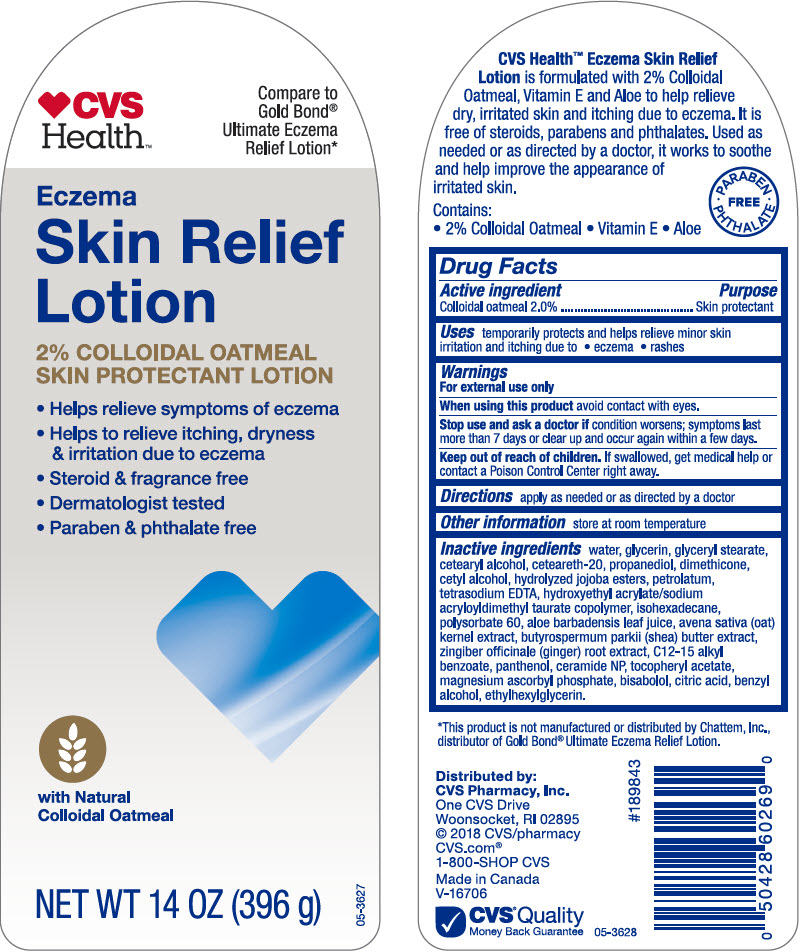
PRINCIPAL DISPLAY PANEL - 396 g Bottle Label
CVS
Health™Compare to
Gold Bond®
Ultimate Eczema
Relief Lotion*Eczema
Skin Relief
Lotion2% COLLOIDAL OATMEAL
SKIN PROTECTANT LOTION- Helps relieve symptoms of eczema
- Helps to relieve itching, dryness
& irritation due to eczema - Steroid & fragrance free
- Dermatologist tested
- Paraben & phthalate free
with Natural
Colloidal OatmealNET WT 14 OZ (396 g)
05-3627
-
INGREDIENTS AND APPEARANCE
CVS HEALTH ECZEMA SKIN RELIEF SKIN PROTECTANT
oatmeal lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-962 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Oatmeal (UNII: 8PI54V663Y) (Oatmeal - UNII:8PI54V663Y) Oatmeal 20 mg in 1 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Glyceryl Monostearate (UNII: 230OU9XXE4) Cetostearyl Alcohol (UNII: 2DMT128M1S) Polyoxyl 20 Cetostearyl Ether (UNII: YRC528SWUY) Propanediol (UNII: 5965N8W85T) Dimethicone (UNII: 92RU3N3Y1O) Cetyl Alcohol (UNII: 936JST6JCN) Hydrolyzed Jojoba Esters (Acid Form) (UNII: UDR641JW8W) Petrolatum (UNII: 4T6H12BN9U) Edetate Sodium (UNII: MP1J8420LU) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) Isohexadecane (UNII: 918X1OUF1E) Polysorbate 60 (UNII: CAL22UVI4M) Aloe Vera Leaf (UNII: ZY81Z83H0X) Oat (UNII: Z6J799EAJK) Shea Butter (UNII: K49155WL9Y) Ginger (UNII: C5529G5JPQ) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Panthenol (UNII: WV9CM0O67Z) Ceramide NP (UNII: 4370DF050B) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) Magnesium Ascorbyl Phosphate (UNII: 0R822556M5) Levomenol (UNII: 24WE03BX2T) Citric Acid Monohydrate (UNII: 2968PHW8QP) BENZYL ALCOHOL (UNII: LKG8494WBH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-962-01 396 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 12/03/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part347 12/03/2018 Labeler - CVS Health (062312574) Registrant - Garcoa Inc. (036464697) Establishment Name Address ID/FEI Business Operations Sigan Industries Inc. 255106239 MANUFACTURE(69842-962)

