Label: SCRUB-STAT- chlorhexidine gluconate solution
-
NDC Code(s):
47593-463-11,
47593-463-21,
47593-463-26,
47593-463-31, view more47593-463-32, 47593-463-33, 47593-463-41, 47593-463-44, 47593-463-56, 47593-463-59
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
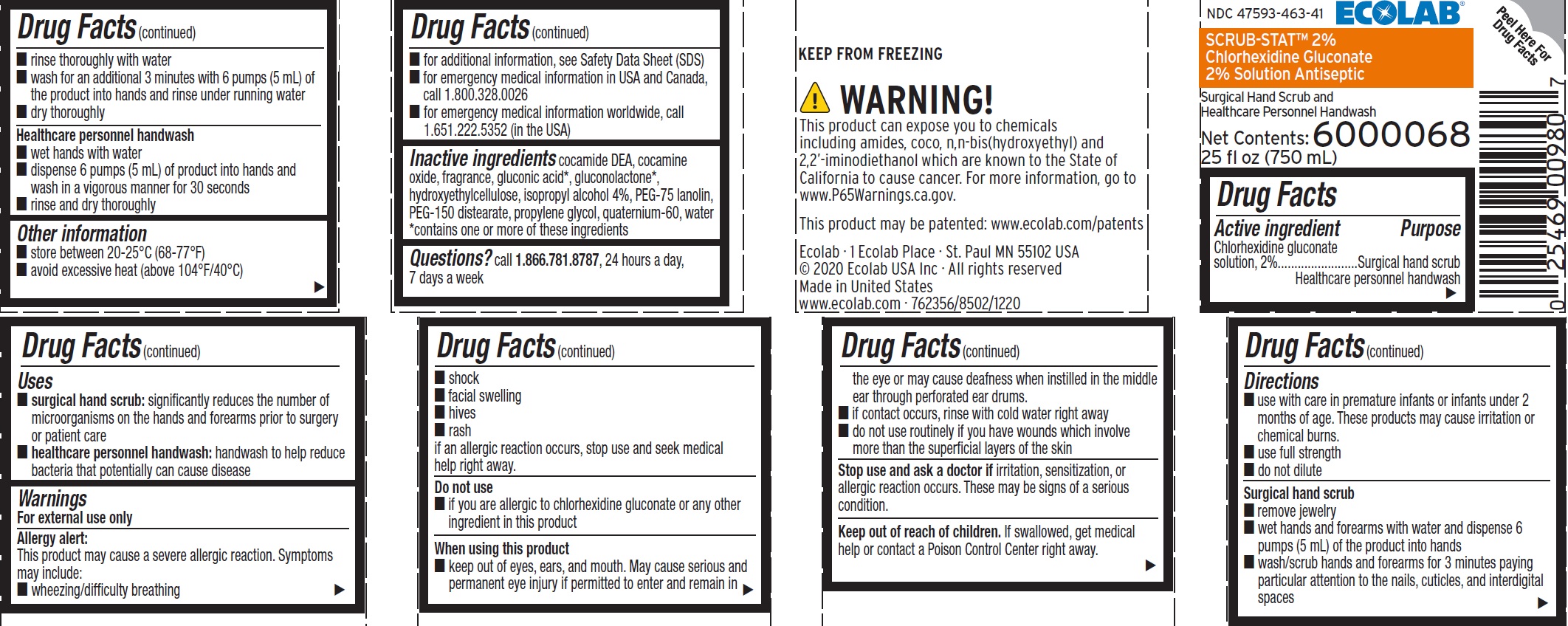
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Allergy alert:
This product may cause a severe allergic reaction.
Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
if an allergic reaction occurs, stop use and seek medical help right away.
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the eye or may cause deafness when instilled in the middle ear through perforated ear drums.
- if contact occurs, rinse with cold water right away
- do not use routinely if you have wounds which involve more than the superficial layers of the skin
-
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
- use full strength
- do not dilute
Surgical hand scrub
- remove jewelry
- wet hands and forearms with water and dispense 6 pumps (5 mL) of the product onto hands
- wash/scrub hands and forearms for 3 minutes paying particular attention to the nails, cuticles, and interdigital spaces
- rinse thoroughly with water
- wash for an additional 3 minutes with 6 pumps (5 mL) of the product into hands and rinse under running water
- dry thoroughly
Healthcare personnel handwash
- wet hands with water
- dispense 6 pumps (5 mL) of product into hands and wash in a vigorous manner for 30 seconds
- rinse and dry thoroughly
- Other information
- INACTIVE INGREDIENT
- QUESTIONS
-
Representative label and principal display panel
ECOLAB®
NDC 47596-463-41
SCRUB-STAT 2%
Chorhexidine Gluconate 2% Solution Antiseptic
Surgical Hand Scrub and
Healthcare Personnel Handwash
6000068
Net Content:
25 fl oz (750 mL)
KEEP FROM FREEZING
Ecolab • 1 Ecolab Place • St Paul MN 55102 USA
© 2020 Ecolab USA Inc • All rights reserved
Made in United States
www.ecolab.com • 762356/8502/1220
-
INGREDIENTS AND APPEARANCE
SCRUB-STAT
chlorhexidine gluconate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-463 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength COCO DIETHANOLAMIDE (UNII: 92005F972D) COCAMINE OXIDE (UNII: QWA2IZI6FI) GLUCONIC ACID (UNII: R4R8J0Q44B) GLUCONOLACTONE (UNII: WQ29KQ9POT) HYDROXYETHYL CELLULOSE (3000 CPS AT 1%) (UNII: 7Q6P4JN1QT) ISOPROPYL ALCOHOL (UNII: ND2M416302) PEG-75 LANOLIN (UNII: 09179OX7TB) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) QUATERNIUM-33 (UNII: XPS4174QZJ) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-463-33 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/1986 2 NDC:47593-463-31 540 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/1986 3 NDC:47593-463-32 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/1986 4 NDC:47593-463-11 3780 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/1986 5 NDC:47593-463-26 1000 mL in 1 POUCH; Type 0: Not a Combination Product 07/22/1986 11/22/2022 6 NDC:47593-463-56 1200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/1986 7 NDC:47593-463-41 750 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/03/2015 8 NDC:47593-463-59 1250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/03/2015 9 NDC:47593-463-44 1134000 mL in 1 CONTAINER; Type 0: Not a Combination Product 12/23/2015 10/15/2024 10 NDC:47593-463-21 208000 mL in 1 DRUM; Type 0: Not a Combination Product 12/23/2015 10/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019258 07/22/1986 Labeler - Ecolab Inc. (006154611)