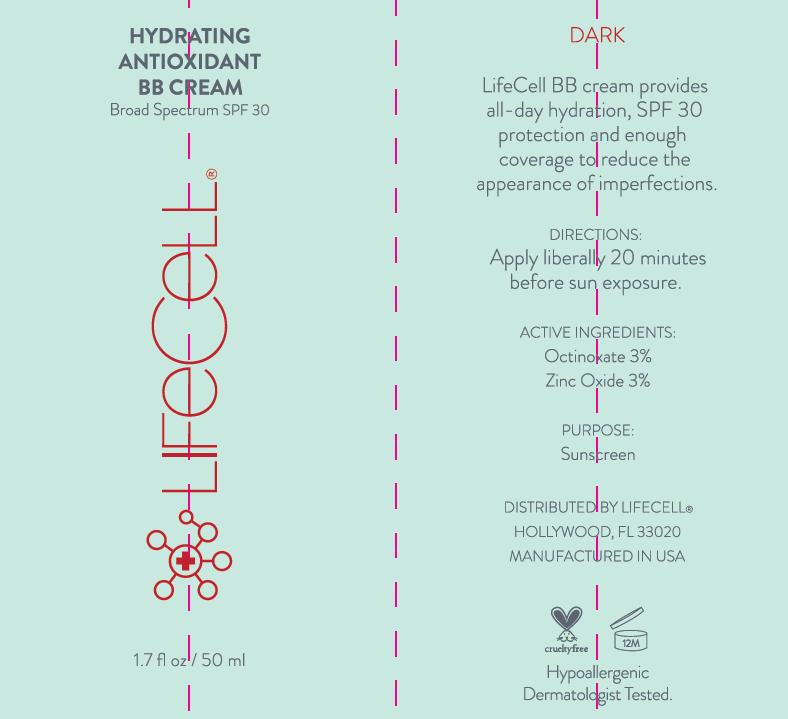
Label: LIFECELL BROAD SPECTRUM SPF 30 BB CREAM- octinoxate and zinc oxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 58443-0191-3 - Packager: Prime Enterprises Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 17, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Uses
- Directions
- Active Ingredients
- PURPOSE
- OTHER SAFETY INFORMATION
- Lifecell BB Cream Broad Spectrum SPF 30
-
INGREDIENTS AND APPEARANCE
LIFECELL BROAD SPECTRUM SPF 30 BB CREAM
octinoxate and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58443-0191 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 29.01 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 29.01 mg in 1 mL Inactive Ingredients Ingredient Name Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALMOND OIL (UNII: 66YXD4DKO9) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BENTONITE (UNII: A3N5ZCN45C) CARAMEL (UNII: T9D99G2B1R) CETYL DIMETHICONE 45 (UNII: IK315POC44) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) CHOLECALCIFEROL (UNII: 1C6V77QF41) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DMDM HYDANTOIN (UNII: BYR0546TOW) HEXYL LAURATE (UNII: 4CG9F9W01Q) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) FERRIC OXIDE RED (UNII: 1K09F3G675) ISOCETYL STEARATE (UNII: 3RJ7186O9W) MELANIN SYNTHETIC (TYROSINE, PEROXIDE) (UNII: O0CV1RMR44) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM (UNII: 7FLD91C86K) OCTYL STEARATE (UNII: 772Y4UFC8B) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) Product Characteristics Color brown Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58443-0191-3 50 mL in 1 TUBE; Type 0: Not a Combination Product 08/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 08/01/2013 Labeler - Prime Enterprises Inc. (101946028) Registrant - Prime Enterprises Inc. (101946028) Establishment Name Address ID/FEI Business Operations Prime Enterprises Inc. 101946028 pack(58443-0191) , manufacture(58443-0191) , label(58443-0191)