Label: NATURASIL MOLLUSCALM TREATMENT KIT- sulfur, thuja occidentalis kit
- NDC Code(s): 10893-810-15, 10893-830-04, 10893-840-02
- Packager: Nature's Innovation, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
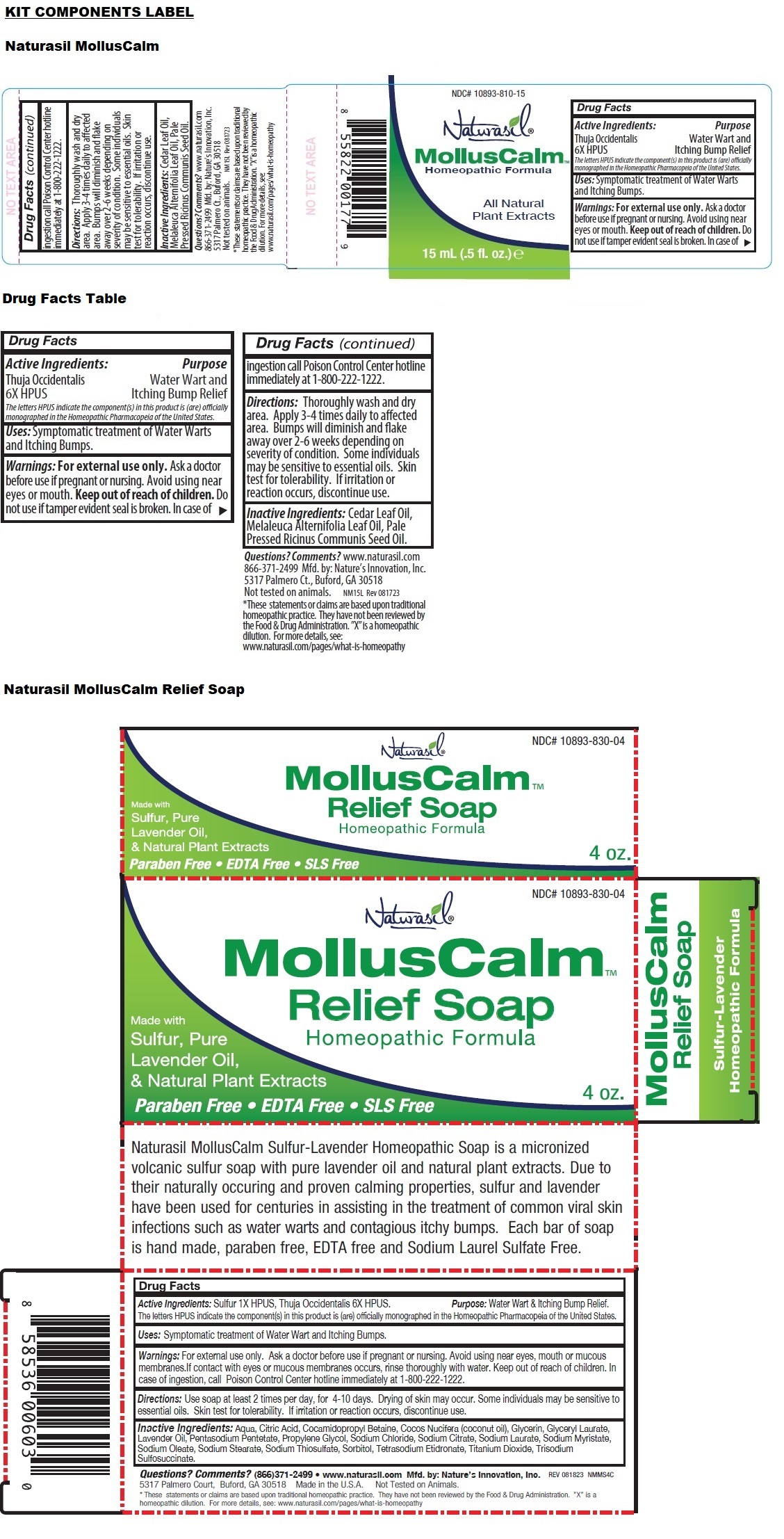
- MollusCalm Liquid
- Active Ingredients:
- Purpose
- Uses:
- Warnings:
- Directions:
- Inactive Ingredients:
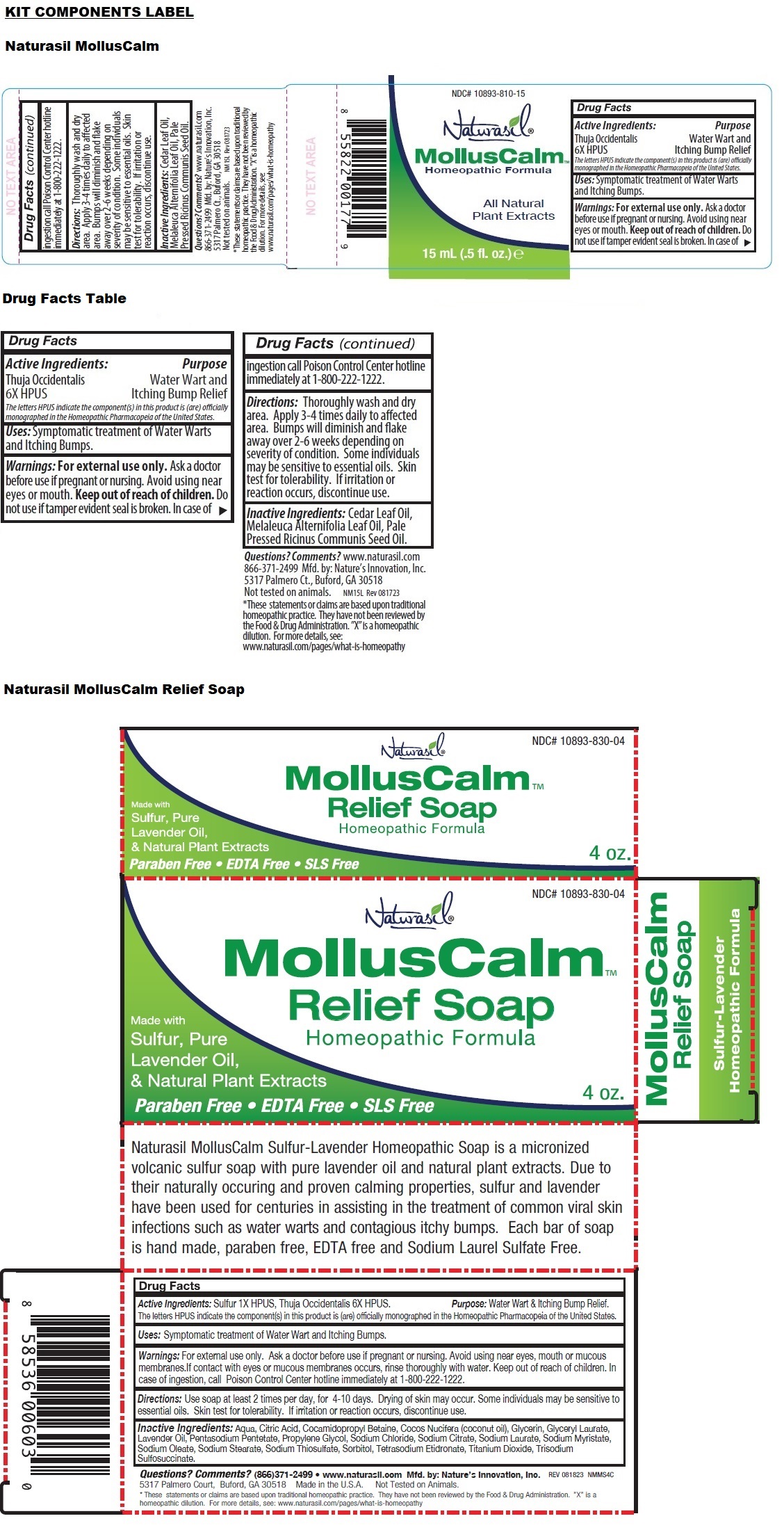
- MollusCalm Soap
- Active Ingredients:
- Purpose:
- Uses:
- Warnings:
- Directions:
-
Inactive Ingredients:
Aqua, Citric Acid, Cocamidopropyl Betaine, Cocos Nucifera (coconut oil), Glycerin, Glyceryl Laurate, Lavender Oil, Pentasodium Pentetate, Propylene Glycol, Sodium Chloride, Sodium Citrate, Sodium Laurate, Sodium Myristate, Sodium Oleate, Sodium Stearate, Sodium Thiosulfate, Sorbitol, Tetrasodium Etidronate, Titanium Dioxide, Trisodium Sulfosuccinate.
-
SPL UNCLASSIFIED SECTION
Homeopathic Formula
All Natural, Homeopathic, No Iodine, Pain-Free for Children and Adults
• Child-Safe (2 Yrs & Above)
• No Acid, Freezing or Burning
• All Natural Ingredients100% GUARANTEE
CRUELTY FREE
NOT TESTED ON ANIMALSEST. IN 2003
1 MIL+ SATISFIED CUSTOMERSIncludes:
MollusCalm Topical Liquid (15mL)
MollusCalm Relief Soap (4 oz.)GMP
GOOD MANUFACTURING PRACTICE
QUALITY PRODUCTMADE IN USA
*These statements or claims are based upon traditional homeopathic practice. They have not been reviewed by the Food & Drug Administration. ”X” is a homeopathic dilution. For more details, see: www.naturasil.com/pages/what-is-homeopathy
Now you can treat those water warts and itching bumps at home with our two-part MollusCalm Treatment Kit. We've included our highly effective 15mL concentrated topical liquid and our 4 oz. formulated soap. Both our concentrated liquid and sulfur-based soap are made with natural plant extracts. These products are made to be Iodine-Free so they are child-safe (2 yrs+). Our products work together effectively to cause the pearl-like nodes to dry up and flake away painlessly over time.
These powerful homeopathic formulas gently clear away the bumps while relieving itching and discomfort, without the pain or cost of chemical laden alternatives. Thuja Occidentalis provides direct relief from water warts often affecting children and adults with lowered immune systems. The Cedar leaf oil, Melaleuca Alternifolia leaf oil and Pale Pressed Ricinus Communis seed oil deliver fast, targeted, pain-free relief.
Naturasil MollusCalm Treatment Kit is recommended as a quick, natural solution that eliminates the need for an expensive dermatologist's visit.
Liquid and soap MUST be used together for optimal relief and healing. Thoroughly wash affect area with MollusCalm Relief Soap and dry. Next, apply MollusCalm Liquid 3-4 times daily directly to the bumps and nodules. Water warts/Pearl-like nodules will diminish and flake away over 2-6 weeks, depending on the severity of the condition.
Questions? Comments?
www.naturasil.com 1-866-371-2499
Mfd. by: Nature’s Innovation, Inc.
5317 Palmero Court, Buford, GA 30518 - Packaging
-
INGREDIENTS AND APPEARANCE
NATURASIL MOLLUSCALM TREATMENT KIT
sulfur, thuja occidentalis kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10893-840 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10893-840-02 1 in 1 KIT; Type 0: Not a Combination Product 08/21/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 15 mL Part 2 1 BOX 118.3 g Part 1 of 2 NATURASIL MOLLUSCALM
thuja occidentalis liquidProduct Information Item Code (Source) NDC:10893-810 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THUJA OCCIDENTALIS LEAF (UNII: 0T0DQN8786) (THUJA OCCIDENTALIS LEAF - UNII:0T0DQN8786) THUJA OCCIDENTALIS LEAF 6 [hp_X] in 15 mL Inactive Ingredients Ingredient Name Strength CEDAR LEAF OIL (UNII: BJ169U4NLG) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) CASTOR OIL (UNII: D5340Y2I9G) Product Characteristics Color yellow Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10893-810-15 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/21/2023 Part 2 of 2 NATURASIL MOLLUSCALM RELIEF
sulfur, thuja occidentalis soapProduct Information Item Code (Source) NDC:10893-830 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 1 [hp_X] in 118.3 g THUJA OCCIDENTALIS LEAF (UNII: 0T0DQN8786) (THUJA OCCIDENTALIS LEAF - UNII:0T0DQN8786) THUJA OCCIDENTALIS LEAF 6 [hp_X] in 118.3 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) COCONUT OIL (UNII: Q9L0O73W7L) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL 1-LAURATE (UNII: WR963Y5QYW) LAVENDER OIL (UNII: ZBP1YXW0H8) PENTASODIUM PENTETATE (UNII: 961TOZ5L7T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM CHLORIDE (UNII: 451W47IQ8X) ANHYDROUS TRISODIUM CITRATE (UNII: RS7A450LGA) SODIUM LAURATE (UNII: K146MR5EXO) SODIUM MYRISTATE (UNII: 06BLC4V0IV) SODIUM OLEATE (UNII: 399SL044HN) SODIUM STEARATE (UNII: QU7E2XA9TG) SODIUM THIOSULFATE ANHYDROUS (UNII: L0IYT1O31N) SORBITOL (UNII: 506T60A25R) ETIDRONATE TETRASODIUM (UNII: CZZ9T1T1X4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRISODIUM SULFOSUCCINATE (UNII: 085WB9L09N) Product Characteristics Color yellow Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10893-830-04 118.3 g in 1 BOX; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/21/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/21/2023 Labeler - Nature's Innovation, Inc. (602969854) Establishment Name Address ID/FEI Business Operations Nature's Innovation, Inc. 602969854 manufacture(10893-840, 10893-810, 10893-830)