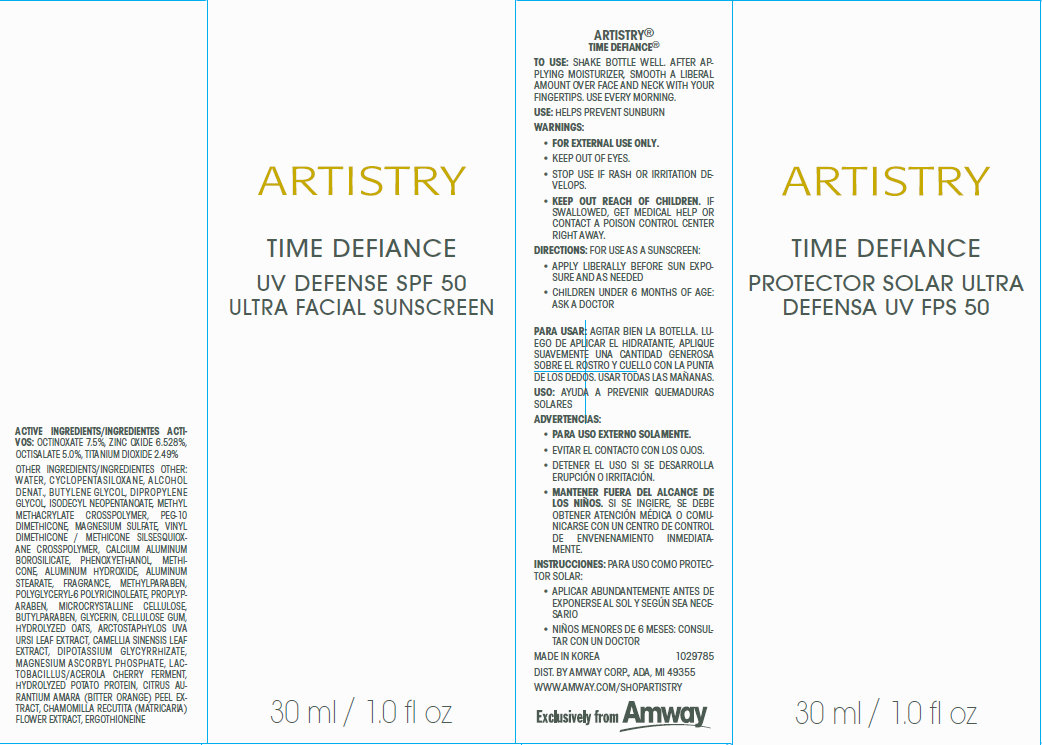
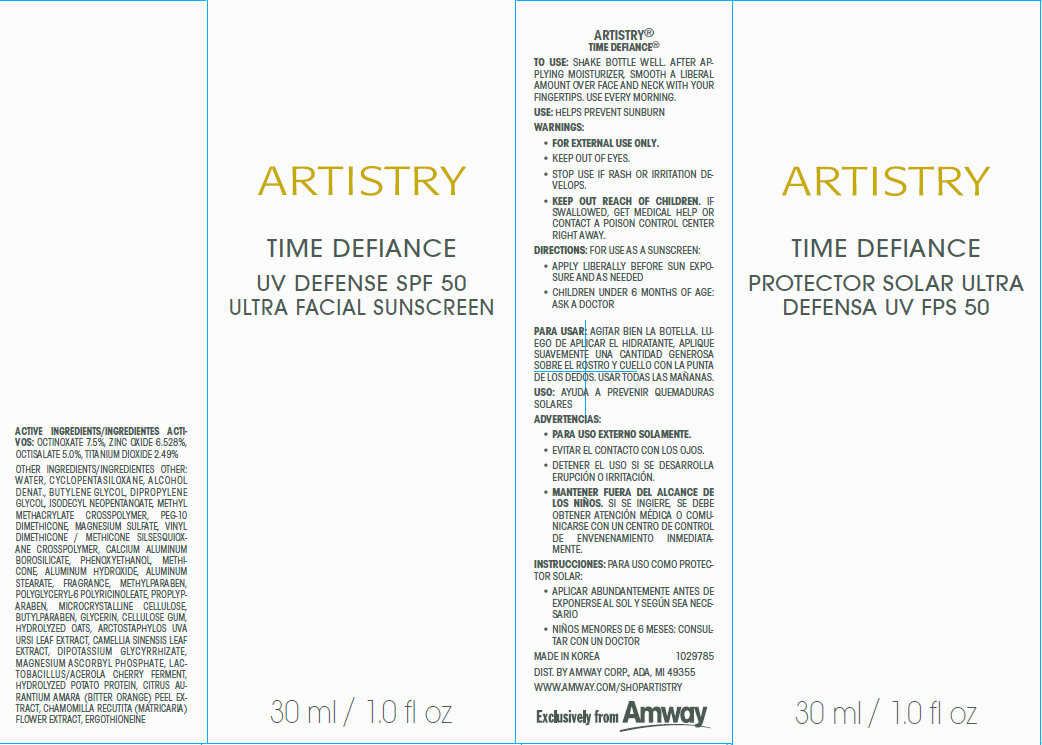
Label: ARTISTRY TIME DEFIANCE UV DEFENSE SPF 50 ULTRA FACIAL SUNSCREEN- octinoxate, zinc oxide, octisalate,titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 50390-110-30 - Packager: Amway Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 17, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ARTISTRY Time Defiance UV Defense SPF 50 Ultra Facial Sunscreen
- Active Ingredients
-
Other Ingredients
WATER , CYCLOPENTASILOXANE , ALCOHOL DENAT., BUTYLENE GLYCOL, DIPROPYLENE GLYCOL, ISODECYL NEOPENTANOATE, METHYL METHACRYLATE CROSSPOLYMER, PEG-10 DIMETHICONE, MAGNESIUM SULFATE, VINYL DIMETHICONE / METHI CONE SILSESQUIOXANE CROSSPOLYMER, CALCIUM ALUMINUM BOROSILI CATE , PHENOXYETHANOL , METHICONE , ALUMINUM HYDROXIDE , ALUMINUM STEARATE, FRAGRANCE, METHYLPARABEN, POLYGLY CERYL -6 POLYRI CINOLEATE , PROPLYPARABEN, MICROCRYSTALLINE CELLULOSE, BUTYLPARABEN, GLYCERIN, CELLULOSE GUM, HYDROLYZED OATS, ARCTOSTAPHYLOS UVA
URSI LEAF EXTRA CT, CAMELLIA SINENSIS LEAF EXTRA CT, DIPOTASSIUM GLY CYRRHI ZATE, MAGNESIUM ASCORBYL PHOSPHATE, LACTOBACILLUS/ ACEROLA CHERRY FERMENT, HYDROLYZED POTATO PROTEIN, CITRUS AURANTIUM AMARA (BITTER ORANGE ) PEEL EXTRACT, CHAMOMILLA RECUTITA (MATRICARIA), FLOWER EXTRACT, ERGOTHIONEINE
- To Use:
- Use:
- Warnings:
- Directions:
- MADE IN KOREA 1029785 DIST. BY AMWAY CORP., ADA, MI 49355 WWW.AMWAY.COM/SHOPARTISTRY
-
ARTISTRY®
TIME DEFIANCE®
UV DEFENSE SPF 50 ULTRA FACIAL SUNSCREEN
Help keep your skin looking younger longer by protecting it with this high-level sunscreen. Moisturizing and fast absorbing, it works during the highest levels of sun exposure to deflect damaging rays and environmental assaults.
Shields skin against dark spots and discoloration. An exclusive Balanced Brightening Complex of 10 extracts, including white tea extract, helps give your skin a luminous glow. This lightweight, water-resistant formula creates the perfect base for makeup to glide on.
To use: Shake bottle well. After applying moisturizer, smooth a liberal amount over face and neck with your fingertips. Use every morning.
Smooth on as the final step in your ARTISTRY TIME DEFIANCE skincare regimen and before applying foundation.
Dermatologist- and allergy-tested. Suitable for use on sensitive skin. Non-acnegenic.
- ARTISTRY Time Defiance UV Defense SPF 50 Ultra Facial Sunscreen 30ml/1oz (50390-110-30)
-
INGREDIENTS AND APPEARANCE
ARTISTRY TIME DEFIANCE UV DEFENSE SPF 50 ULTRA FACIAL SUNSCREEN
octinoxate, zinc oxide, octisalate,titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50390-110 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 mL in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.528 mL in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 mL in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.49 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIPROPYLENE GLYCOL (UNII: E107L85C40) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) METHYL METHACRYLATE (UNII: 196OC77688) MAGNESIUM SULFATE (UNII: DE08037SAB) CALCIUM ALUMINOSILICATE (UNII: 3L00JH8411) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHICONE (20 CST) (UNII: 6777U11MKT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM STEARATE (UNII: U6XF9NP8HM) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) BUTYLPARABEN (UNII: 3QPI1U3FV8) GLYCERIN (UNII: PDC6A3C0OX) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) OAT (UNII: Z6J799EAJK) ARCTOSTAPHYLOS UVA-URSI LEAF (UNII: 3M5V3D1X36) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) ACEROLA (UNII: XDD2WEC9L5) POTATO (UNII: CFE1S8DYWD) CITRUS AURANTIUM FRUIT RIND (UNII: 055456JHI7) CHAMOMILE (UNII: FGL3685T2X) ERGOTHIONEINE (UNII: BDZ3DQM98W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50390-110-30 30 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/10/2012 Labeler - Amway Corp (083416854) Establishment Name Address ID/FEI Business Operations Kolmar Korea Co. Ltd. 687846360 manufacture