Label: KOPERTOX- copper naphthenate liquid
- NDC Code(s): 54771-1100-1, 54771-1100-2
- Packager: Zoetis
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated December 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
- PRECAUTIONS
- ACTIVE INGREDIENT:
- INACTIVE INGREDIENTS:
- PRECAUTIONS
- INDICATIONS & USAGE
-
GENERAL PRECAUTIONS
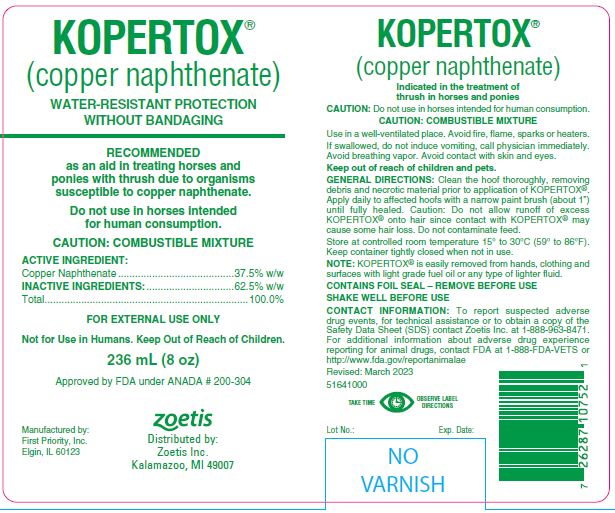
CAUTION: Do not use in horses intended for human consumption.
CAUTION: COMBUSTIBLE MIXTURE
Use in a well-ventilated place. Avoid fire, flame, sparks or heaters.
If swallowed, do not induce vomiting, call physician immediately. Avoid breathing vapor. Avoid contact with skin and eyes.
Keep out of reach of children and pets.
-
GENERAL DIRECTIONS:
Clean the hoof thoroughly, removing debris and necrotic material prior to application of KOPERTOX®. Apply daily to affected hoofs with a narrow paint brush (about 1") until fully healed. Caution: Do not allow runoff of excess KOPERTOX® onto hair since contact with KOPERTOX® may cause some hair loss. Do not contaminate feed.
- STORAGE AND HANDLING
- INFORMATION FOR OWNERS/CAREGIVERS
-
CONTACT INFORMATION:
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS) contact Zoetis Inc. at
1-888-963-8471. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae
- HOW SUPPLIED
- 236 mL (8 oz)
- 473 mL (16 oz)
-
INGREDIENTS AND APPEARANCE
KOPERTOX
copper naphthenate liquidProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:54771-1100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COPPER NAPHTHENATE (UNII: 9J2IBN2H70) (COPPER - UNII:789U1901C5) COPPER NAPHTHENATE 0.825 g in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-1100-1 12 in 1 CASE 1 236 mL in 1 BOTTLE, DISPENSING 2 NDC:54771-1100-2 12 in 1 CASE 2 473 mL in 1 BOTTLE, DISPENSING Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200304 10/12/2023 Labeler - Zoetis (828851555) Establishment Name Address ID/FEI Business Operations FIRST PRIORITY INCORPORATED 179925722 manufacture