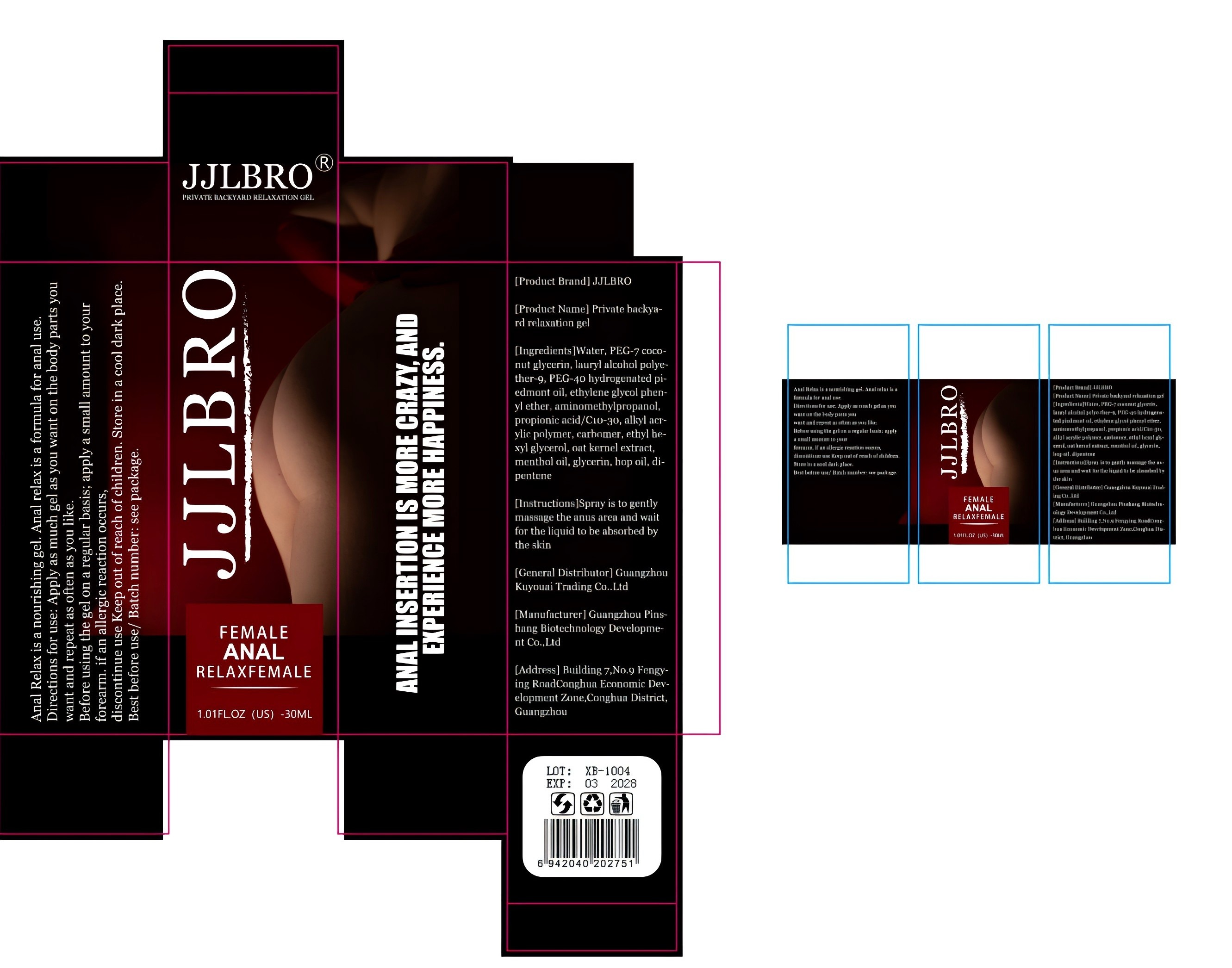
Label: PRIVATE BACKYA-RD RELAXATION GEL gel
- NDC Code(s): 84372-057-01
- Packager: Shenzhen Zhumeng Times Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Contraindications, Precautions, Warnings, and Instructions
- Dosage and administration
- Do not use
- When using section
- Inactive ingredients
- stop use
- Keep out of reach of children.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PRIVATE BACKYA-RD RELAXATION GEL
private backya-rd relaxation gel gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84372-057 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LAURYL ALCOHOL (UNII: 178A96NLP2) (LAURYL ALCOHOL - UNII:178A96NLP2) LAURYL ALCOHOL 5 mg in 5 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) WATER (UNII: 059QF0KO0R) ALKYLSEVIN (UNII: JKS3UE1KPG) MENTHOL, (+)- (UNII: C6B1OE8P3W) PROPIONIC ACID (UNII: JHU490RVYR) HOPS OIL (UNII: 06JI9W529S) N-ETHYLHEXEDRONE (UNII: TAX3KSX6GY) OAT (UNII: Z6J799EAJK) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYDROGENATED RAPESEED OIL (UNII: K168T6Y0YU) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) 2-PENTENE (UNII: Y5RK21VDPE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84372-057-01 30 mg in 1 BOX; Type 1: Convenience Kit of Co-Package 09/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 09/15/2024 Labeler - Shenzhen Zhumeng Times Technology Co., Ltd. (631852731) Establishment Name Address ID/FEI Business Operations Shenzhen Zhumeng Times Technology Co., Ltd. 631852731 manufacture(84372-057)