Label: DR CANUSO FUNGAL NAIL ERASER- tolnaftate oil oil
- NDC Code(s): 76348-410-02
- Packager: Renu Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
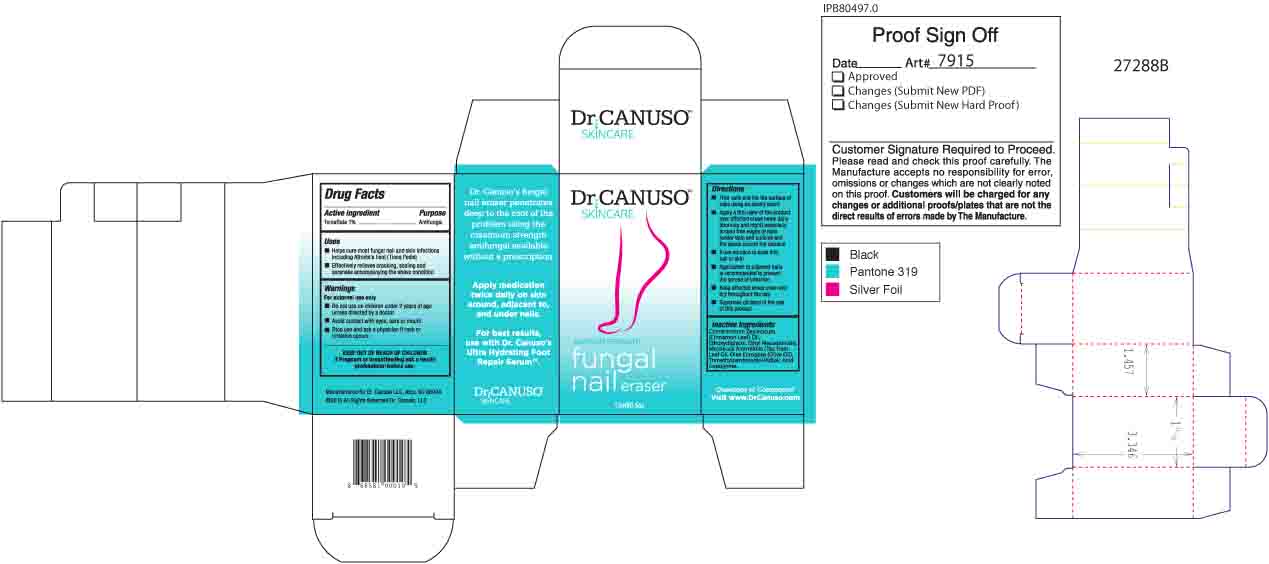
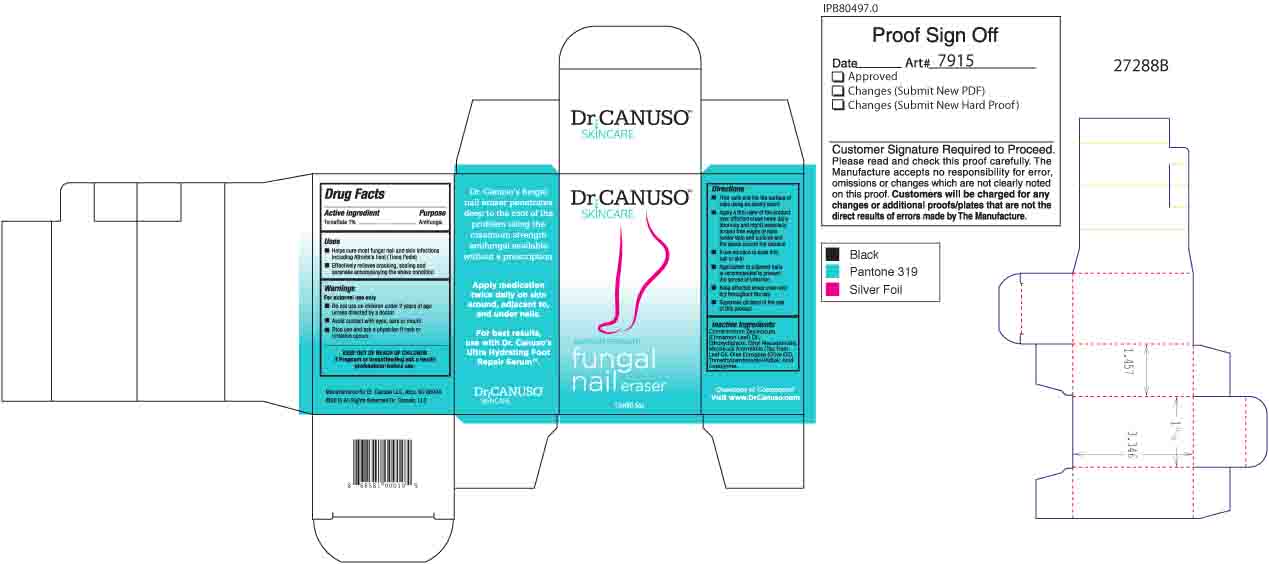
- ACTIVE INGREDIENT
- PURPOSE
- Uses
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
-
Directions
- Trim nails and file the surface of nails using an emory board
- Apply a thin layer of the product over affected areas twice daily (morning and night) especially around free edges of nails (under tips) and cuticles and the space around the toenails.
- Allow solution to soak into nail or skin
- Application to adjacent nails is recommended to prevent the spread of infection
- Keep affected areas clean and dry throughout the day
- Supervise children in the use of this product
- INACTIVE INGREDIENT
- QUESTIONS
- DOSAGE & ADMINISTRATION
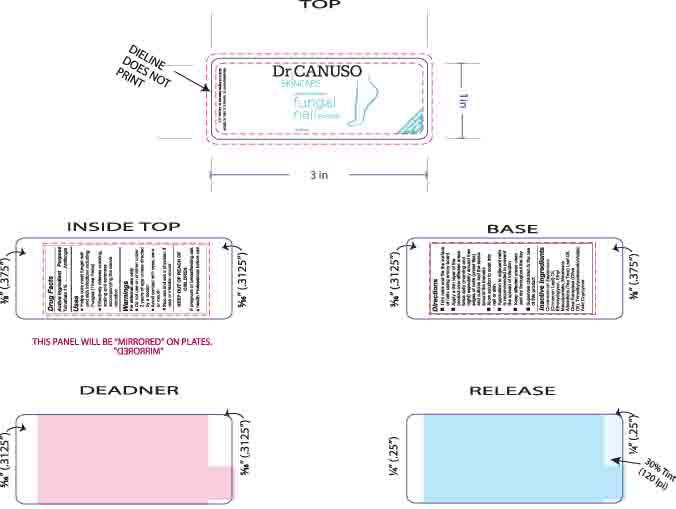
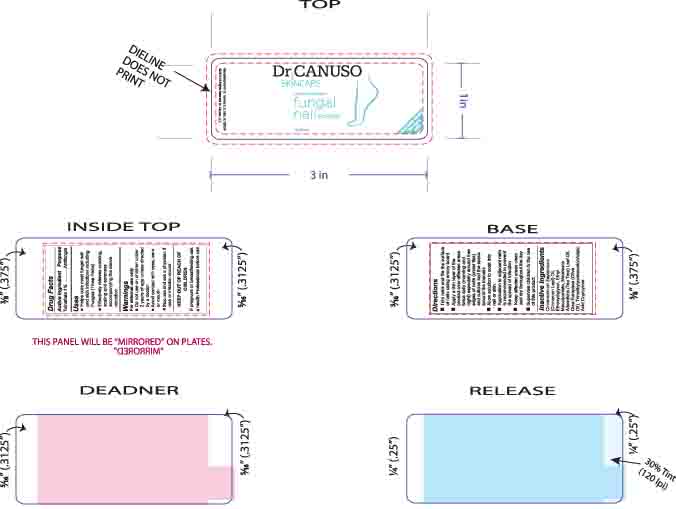
- LABEL FOR BOTTLED PRODUCT
- Product BOX
-
INGREDIENTS AND APPEARANCE
DR CANUSO FUNGAL NAIL ERASER
tolnaftate oil oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76348-410 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 0.3 g in 28 g Inactive Ingredients Ingredient Name Strength ETHYL MACADAMIATE (UNII: ANA2NCS6V1) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) CINNAMON LEAF OIL (UNII: S92U8SQ71V) TEA TREE OIL (UNII: VIF565UC2G) TRIMETHYLPENTANEDIOL/ADIPIC ACID/GLYCERIN CROSSPOLYMER (25000 MPA.S) (UNII: 587WKM3S9Q) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) OLIVE OIL (UNII: 6UYK2W1W1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76348-410-02 1 in 1 BOX 08/11/2015 1 14 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 08/11/2015 Labeler - Renu Laboratories, Inc. (945739449) Establishment Name Address ID/FEI Business Operations Renu Laboratories, Inc. 945739449 manufacture(76348-410)