Label: BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 FAIR- titanium dioxide and zinc oxide liquid
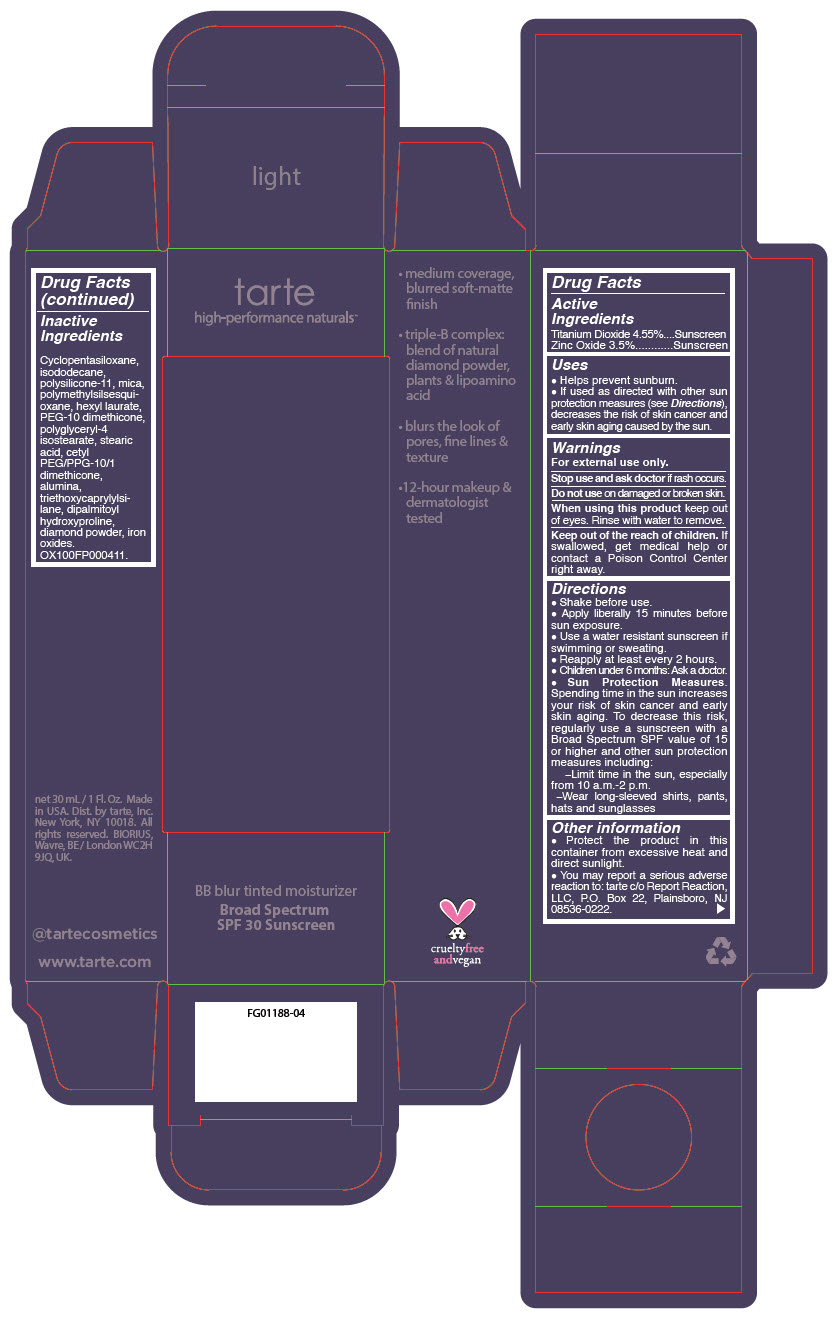
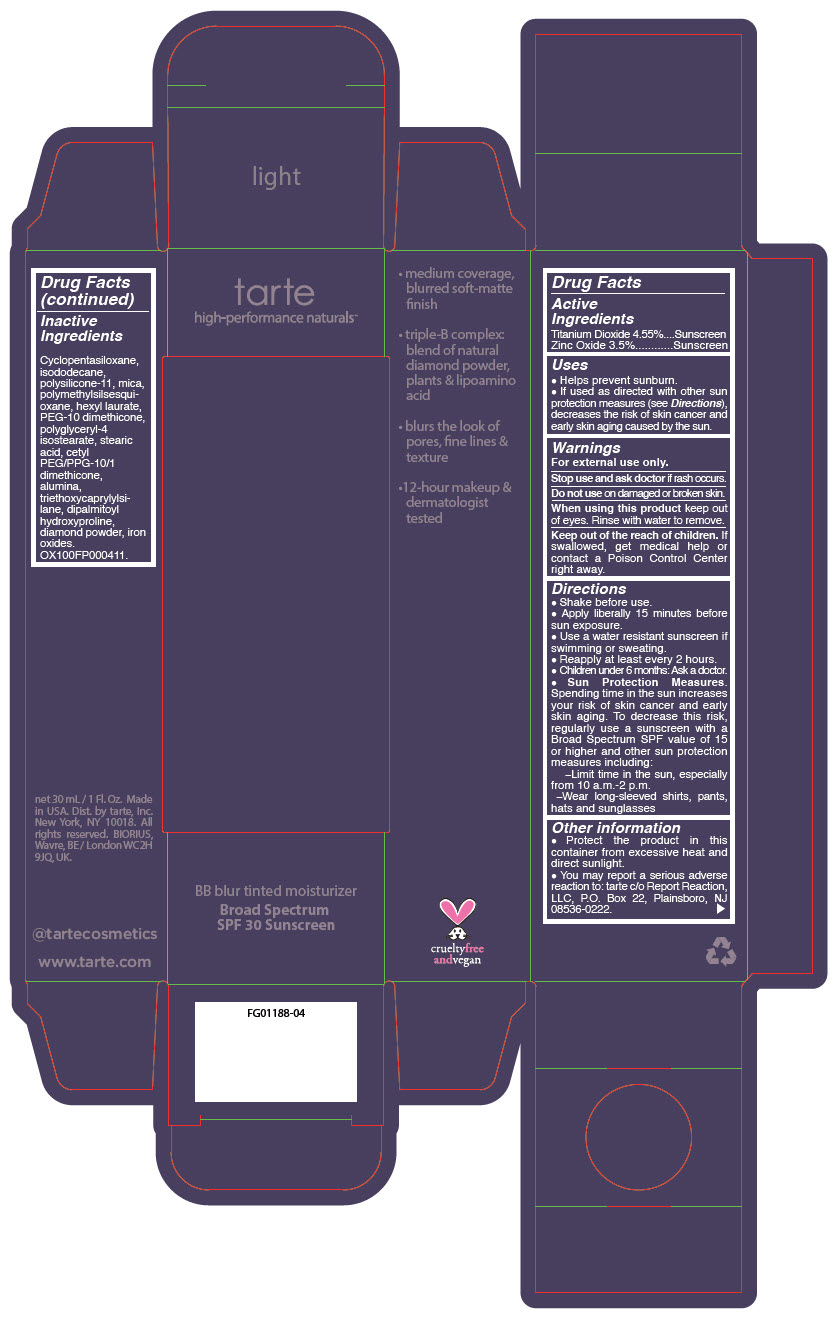
BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 LIGHT- titanium dioxide and zinc oxide liquid
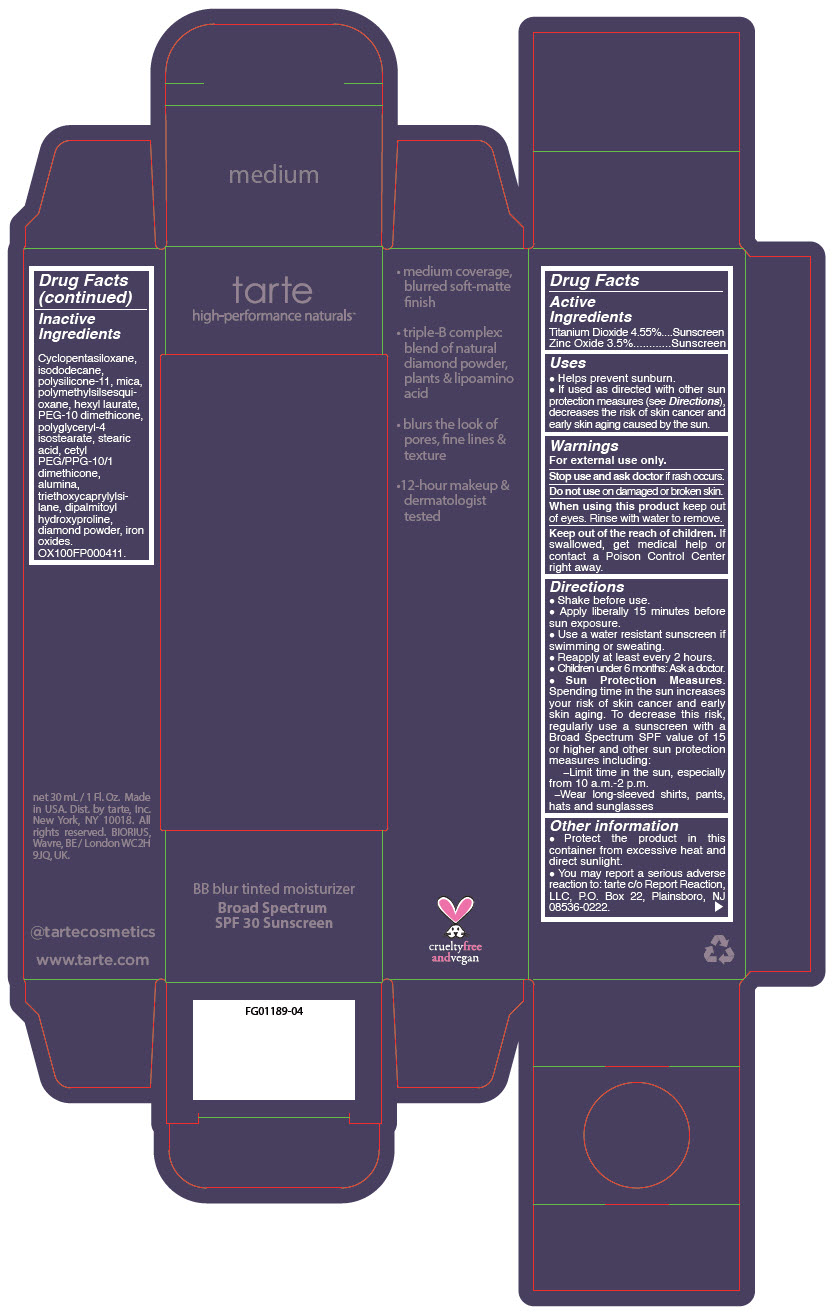
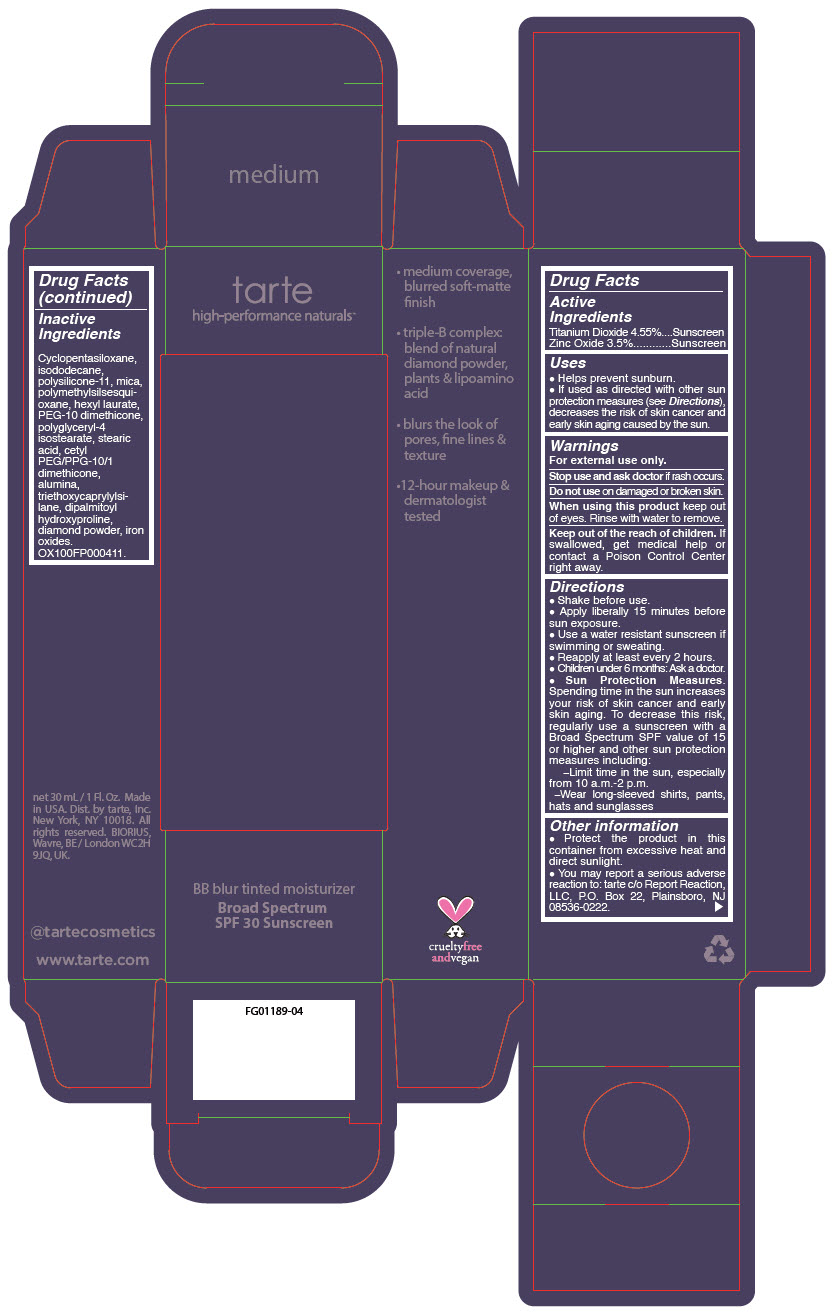
BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 MEDIUM- titanium dioxide and zinc oxide liquid
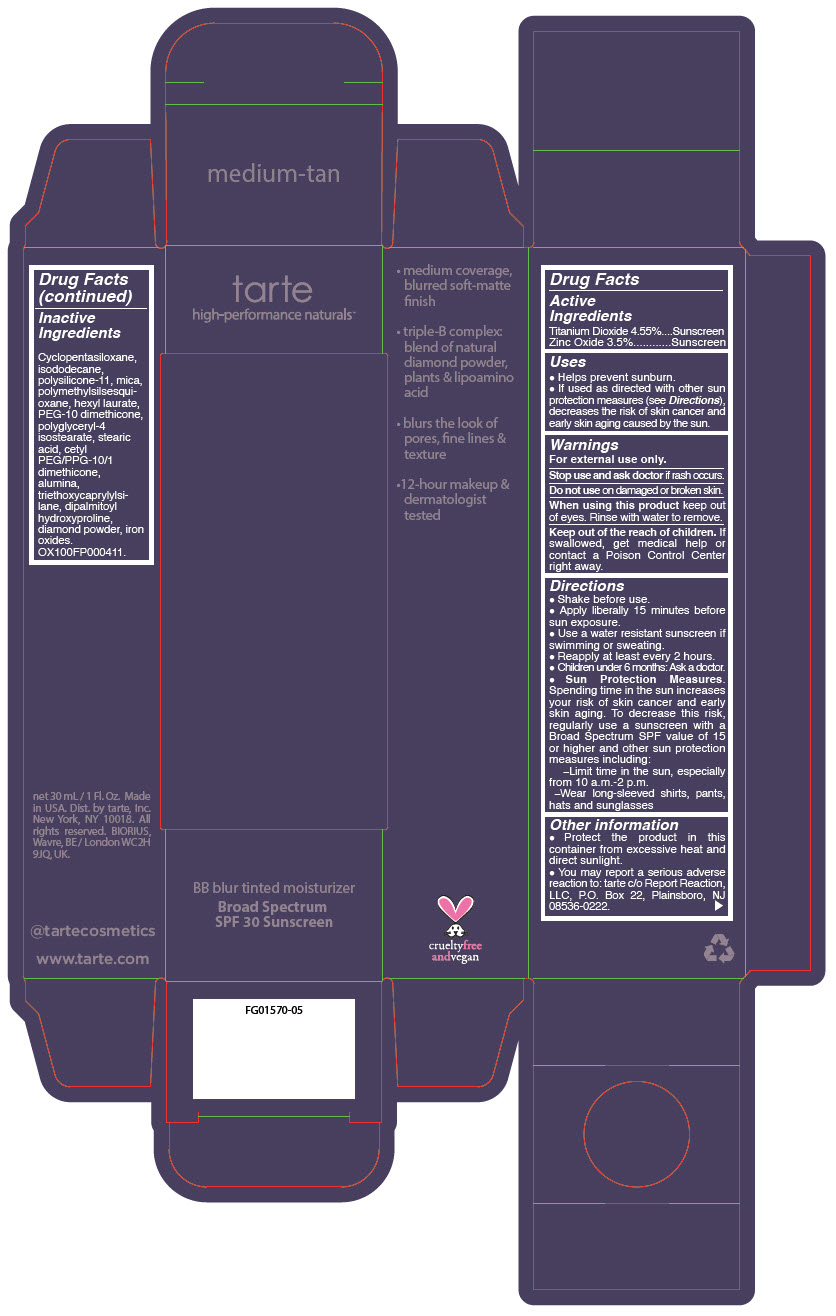
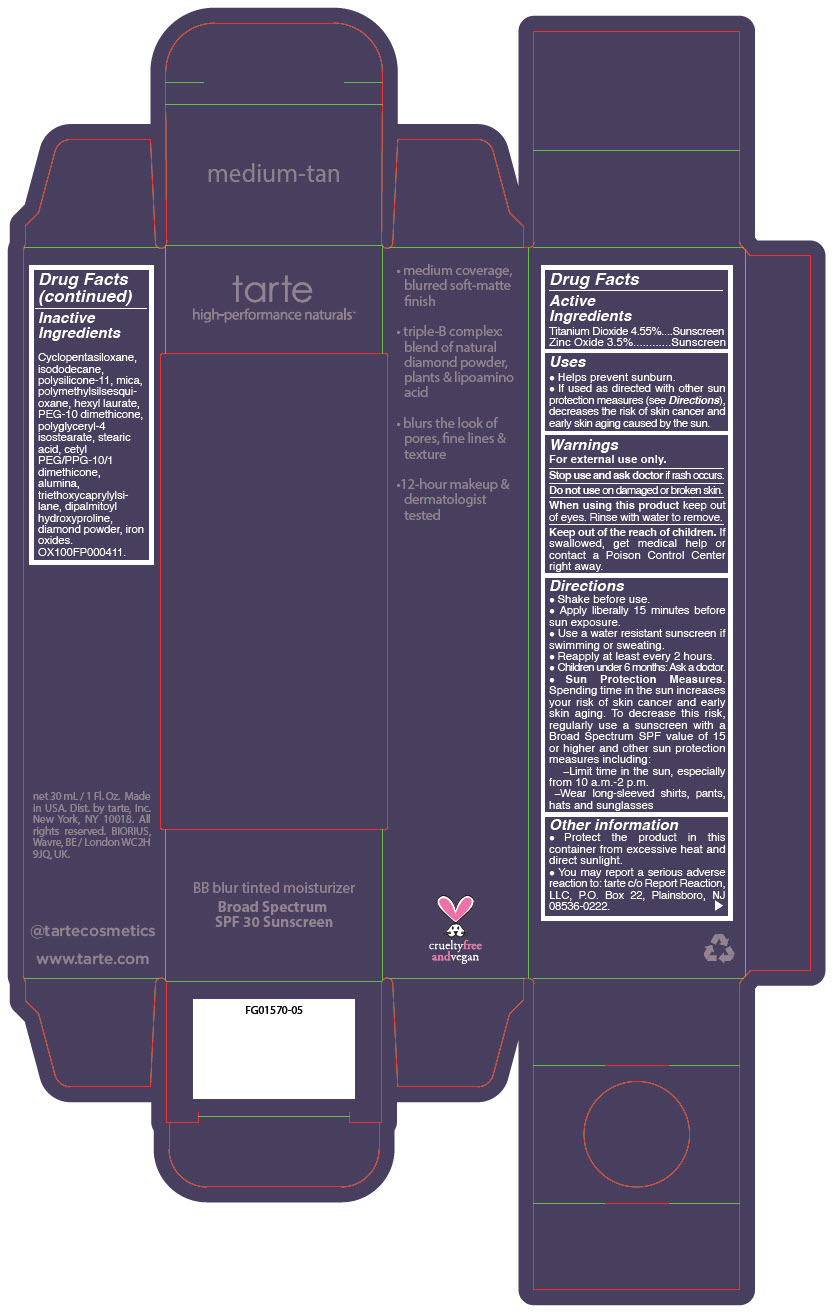
BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 MEDIUM-TAN- titanium dioxide and zinc oxide liquid
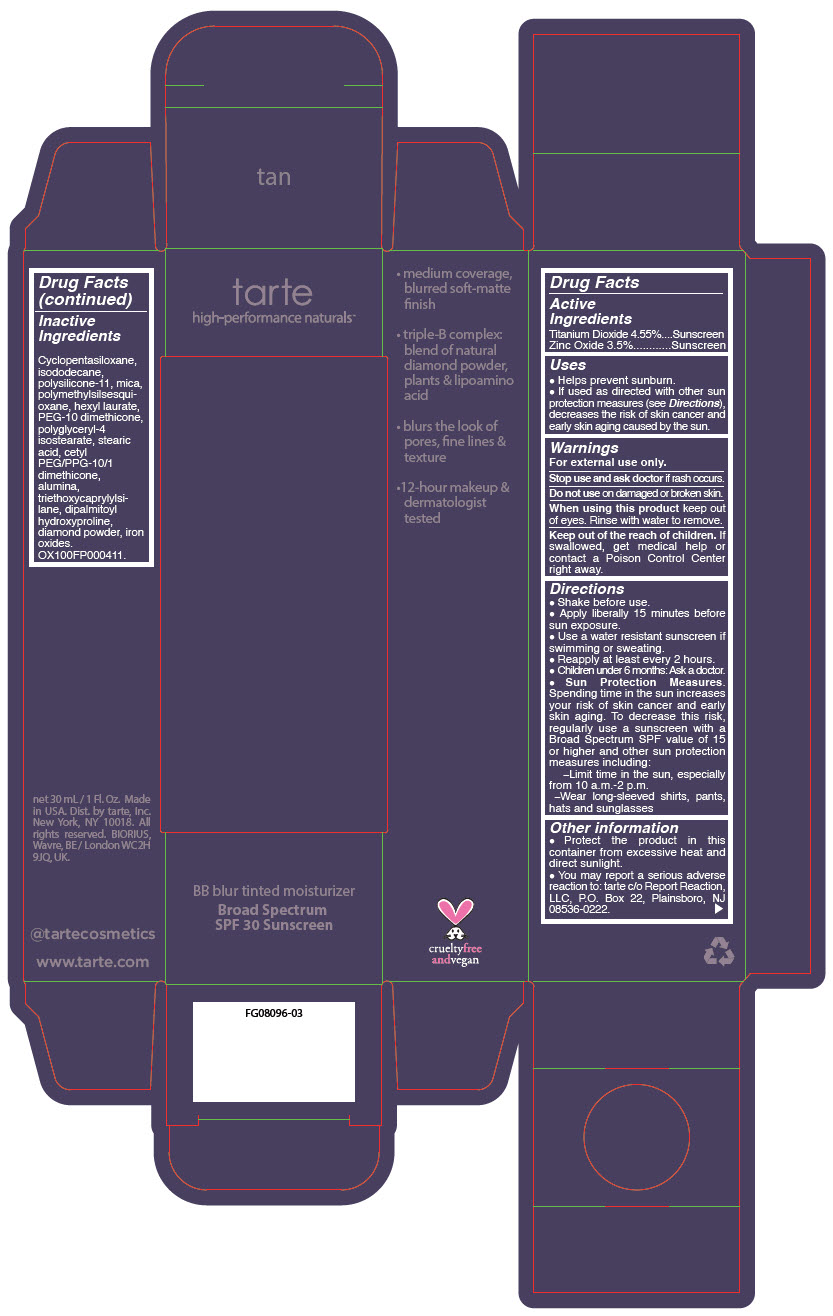
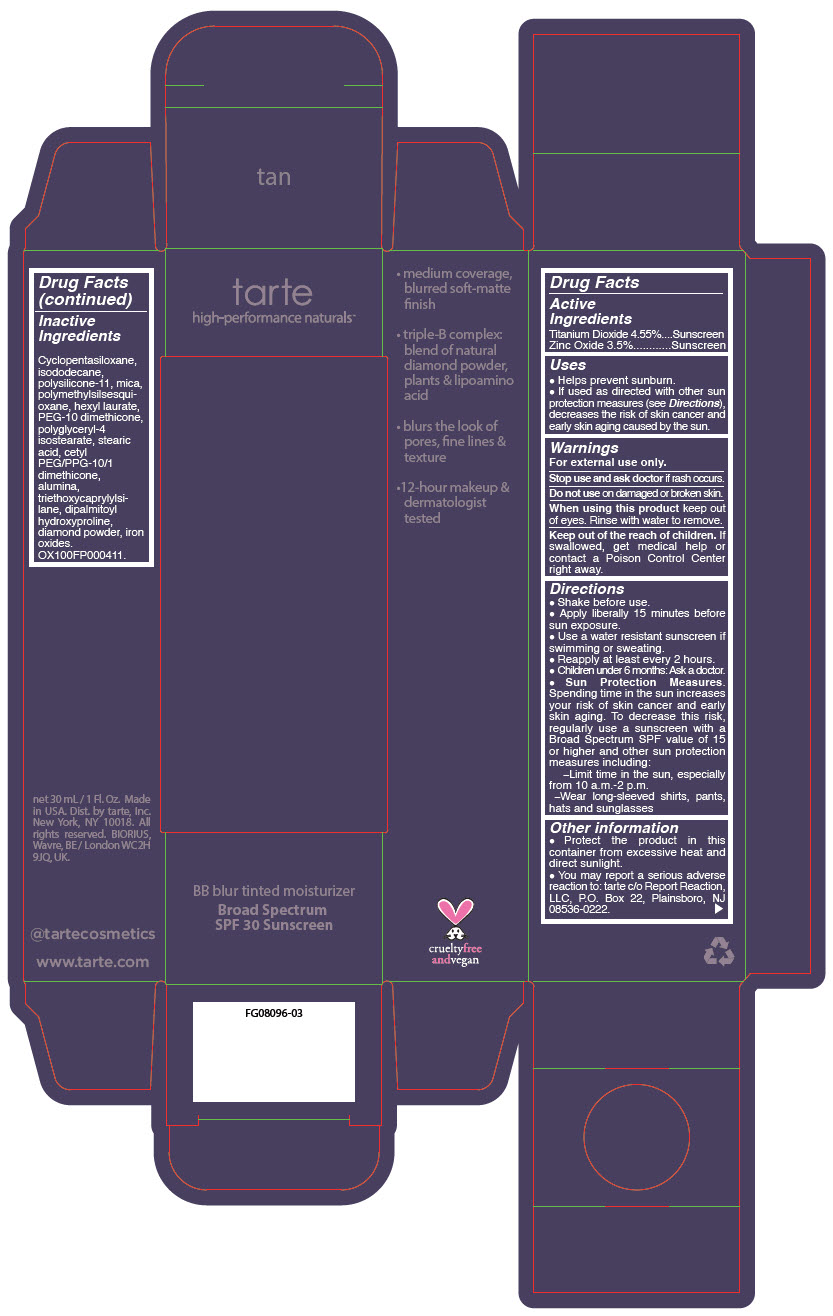
BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 TAN- titanium dioxide and zinc oxide liquid
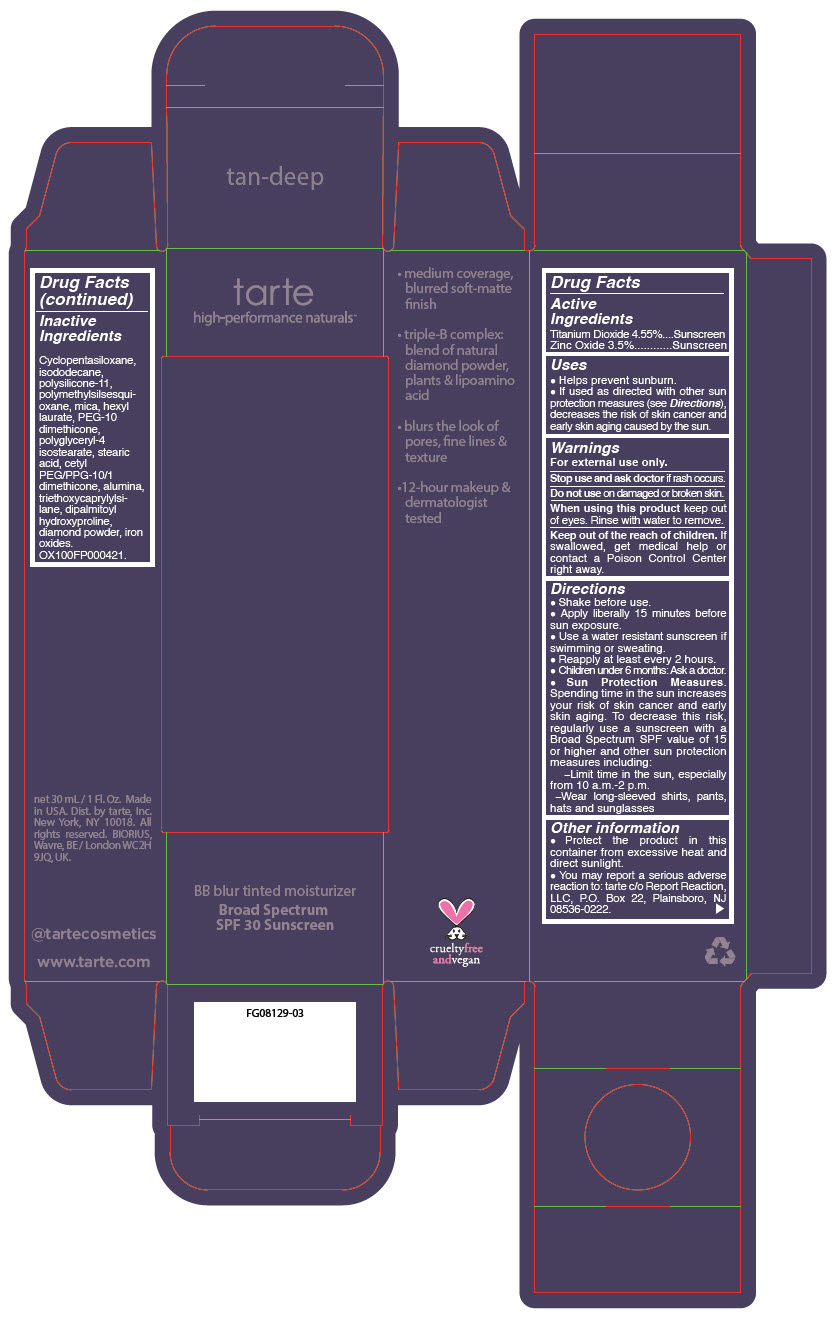
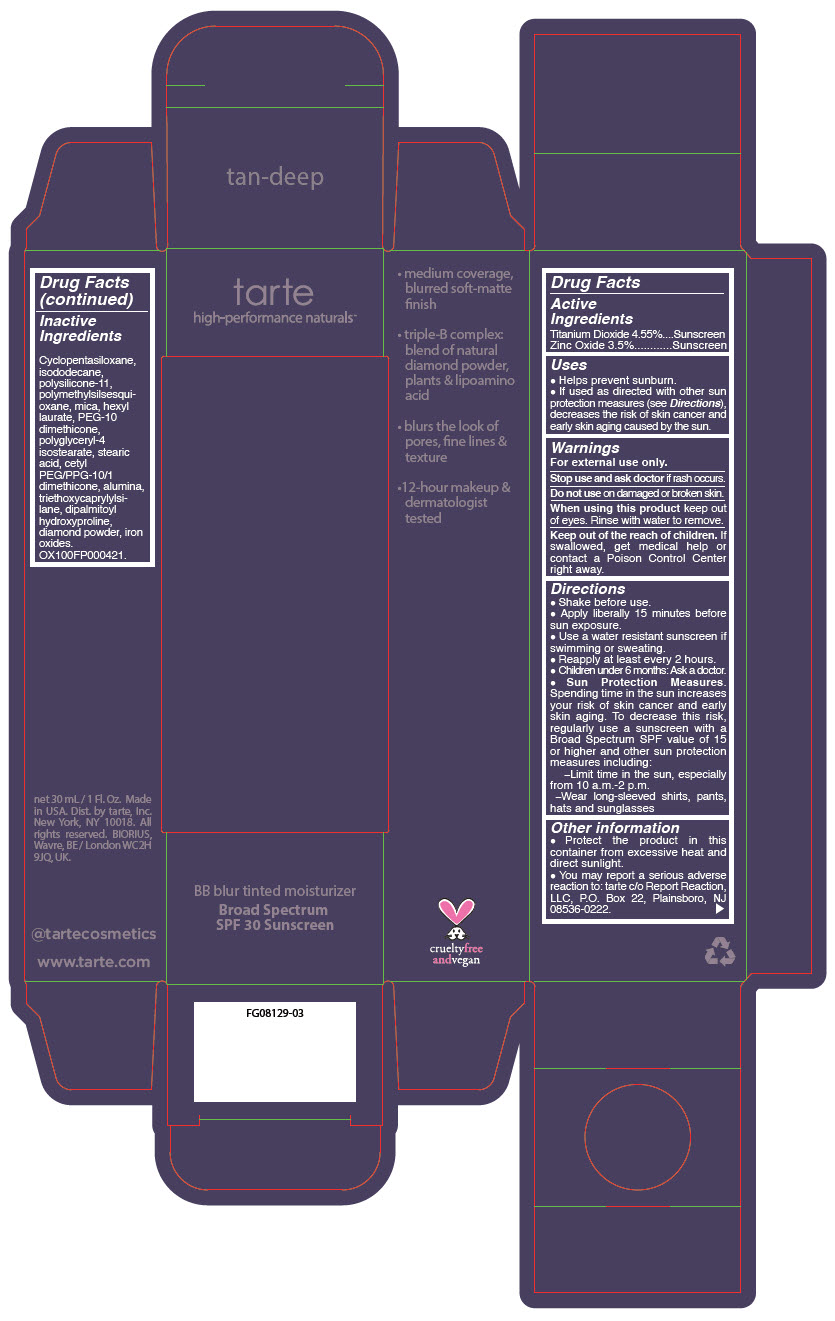
BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 TAN DEEP- titanium dioxide and zinc oxide liquid
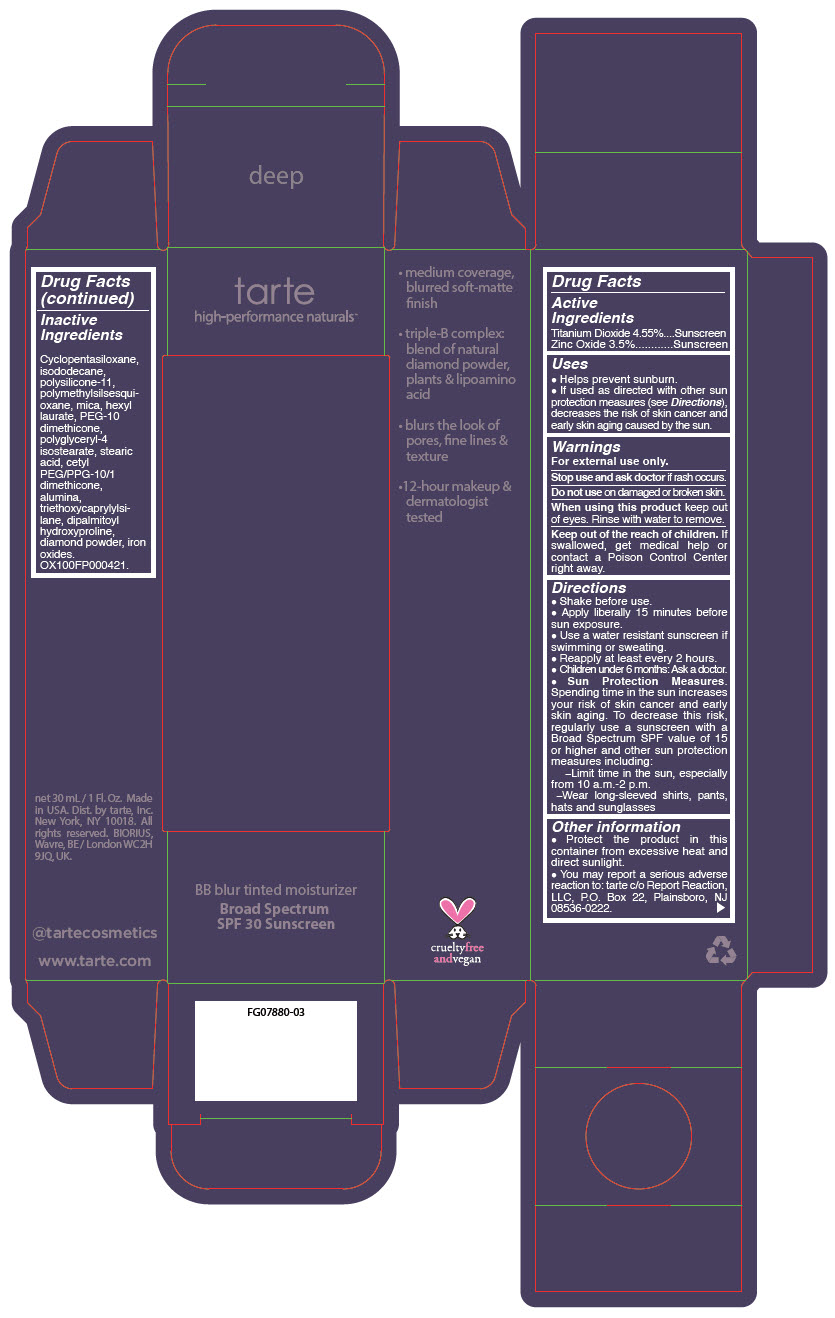
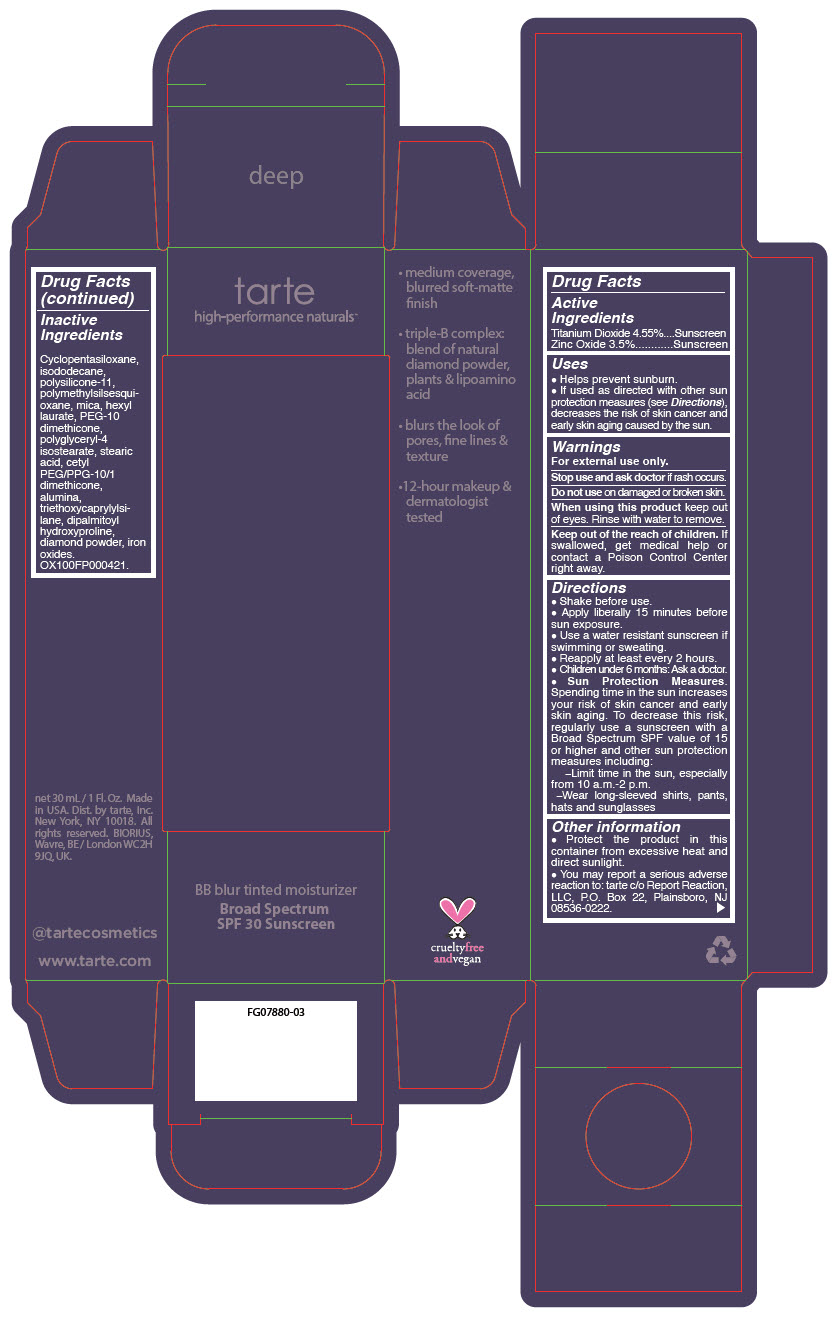
BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 DEEP- titanium dioxide and zinc oxide liquid
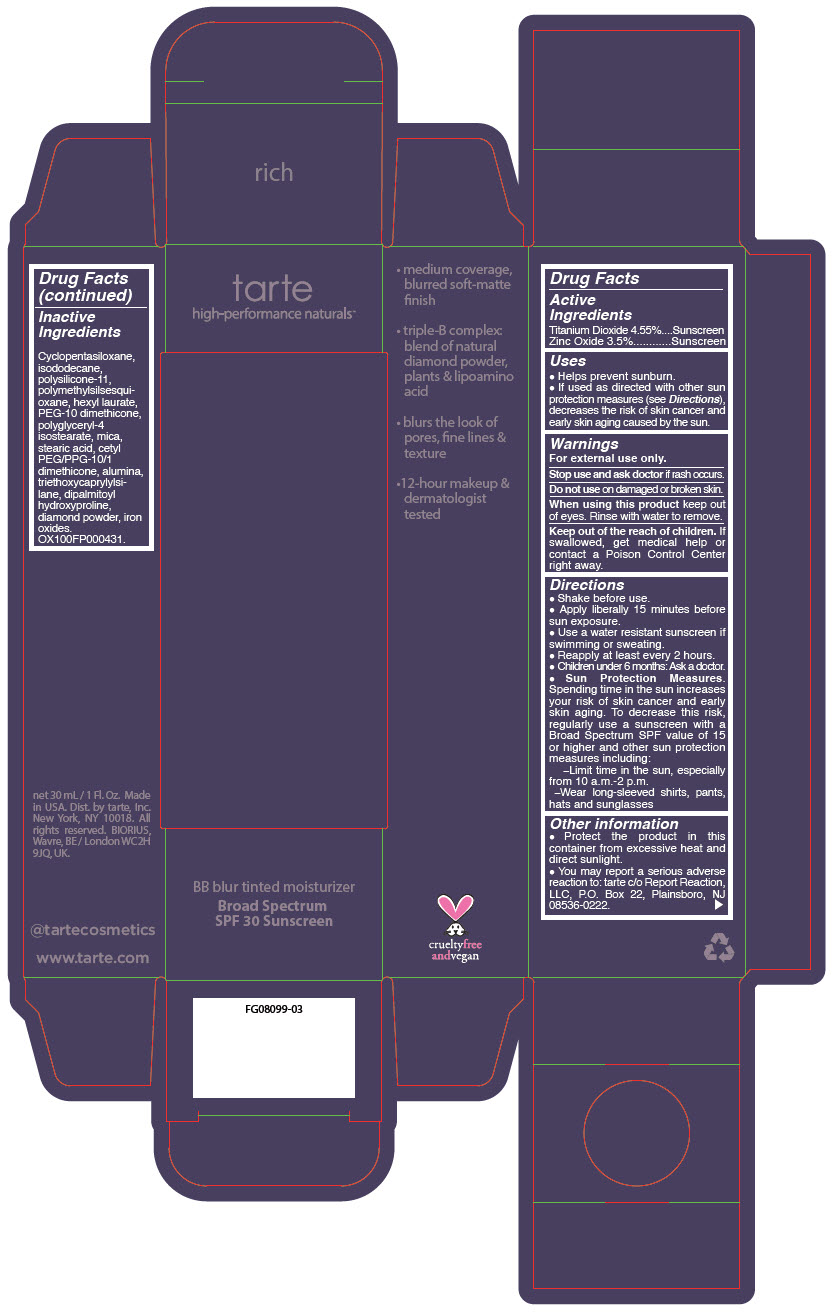
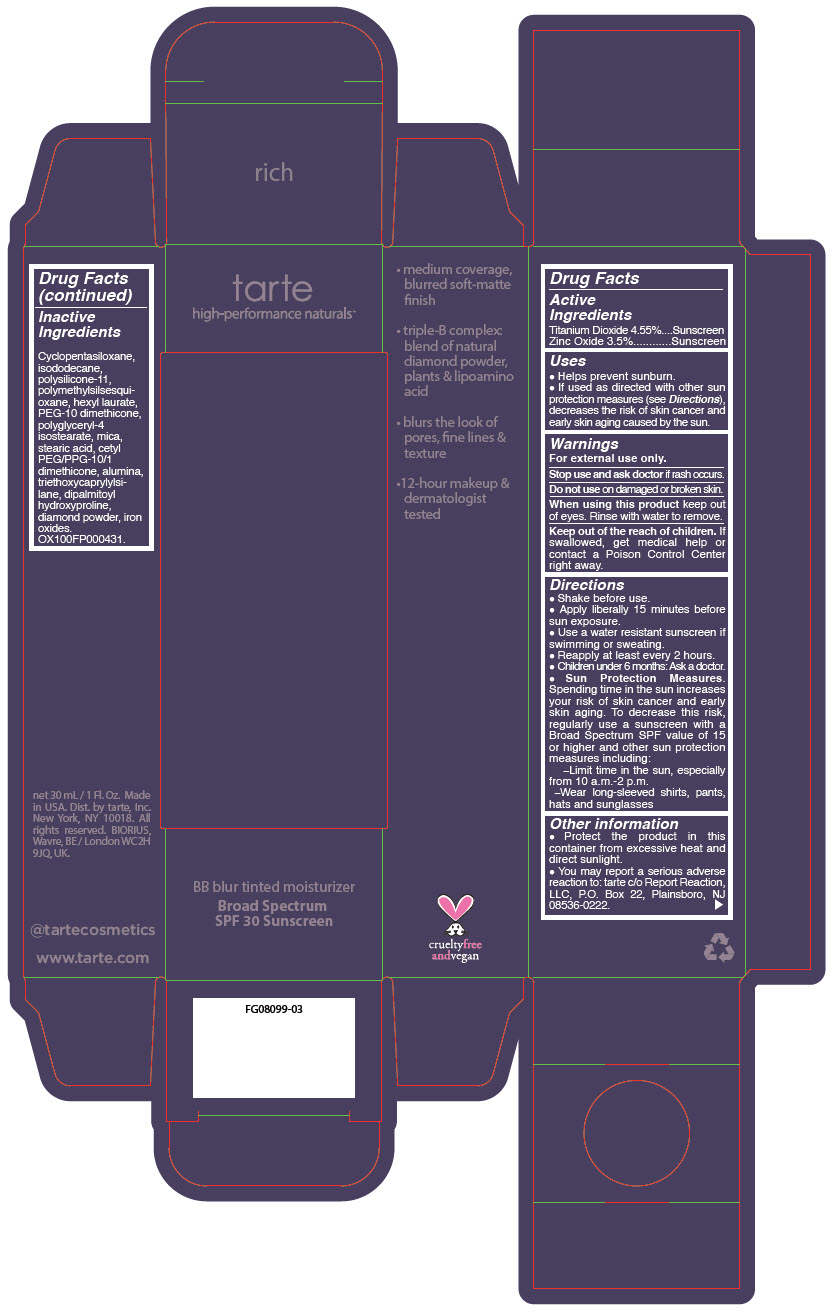
BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 RICH- titanium dioxide and zinc oxide liquid
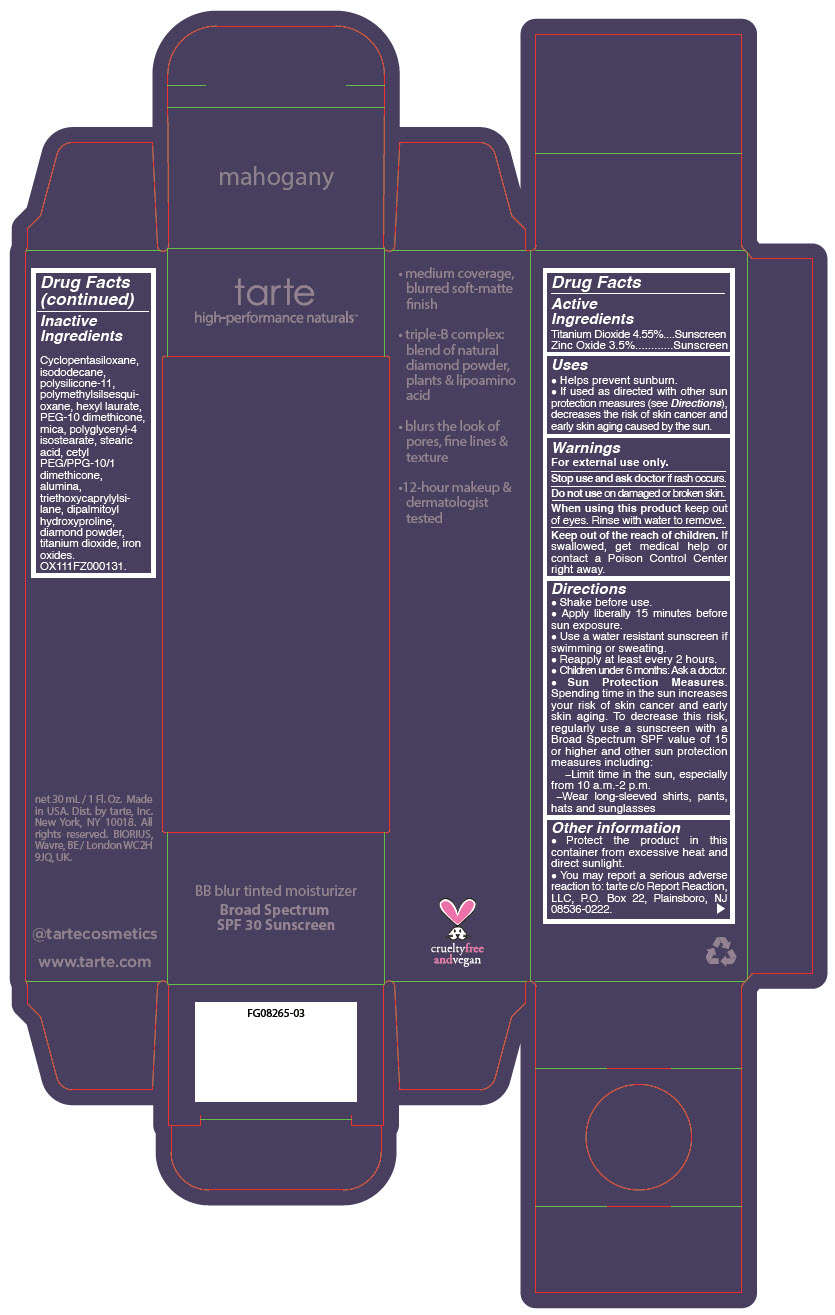
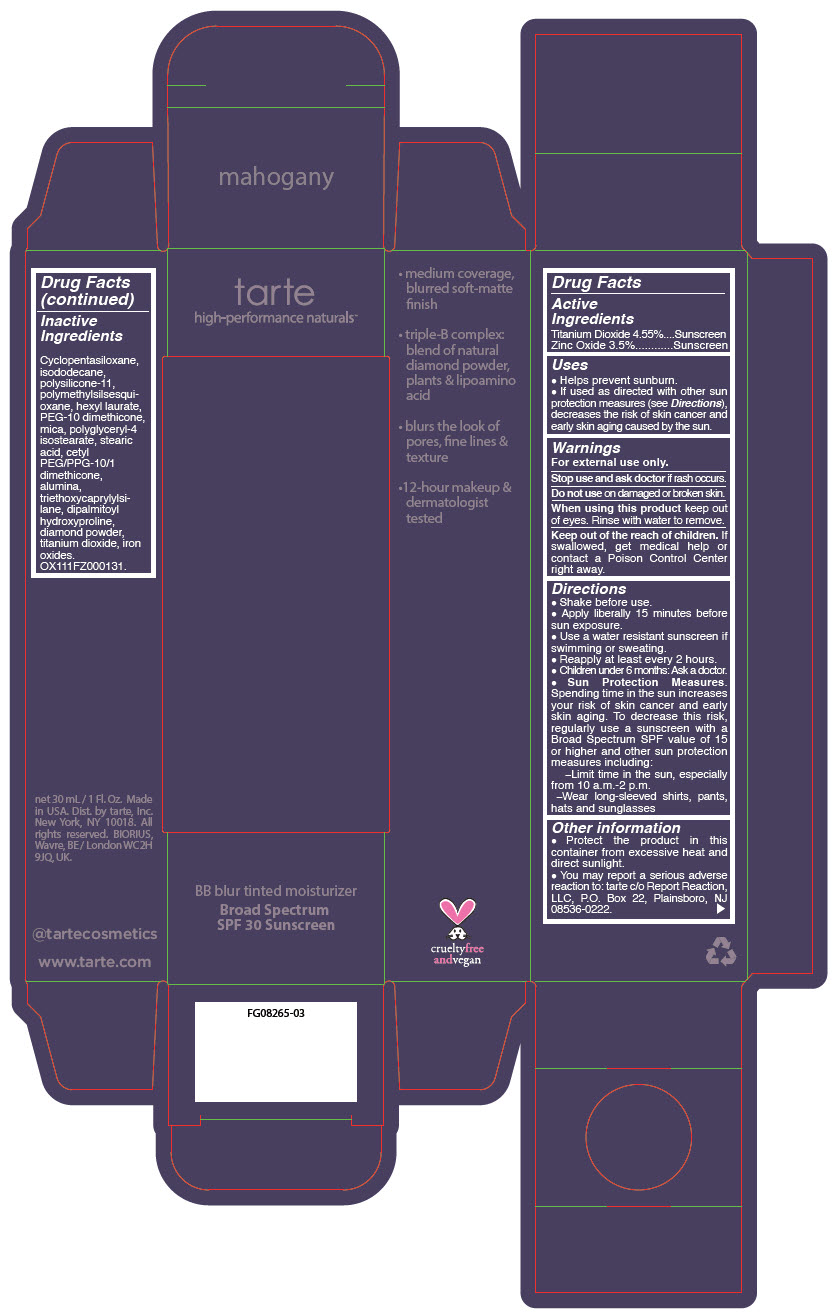
BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 MAHOGANY- titanium dioxide and zinc oxide liquid
-
NDC Code(s):
51060-365-01,
51060-366-01,
51060-367-01,
51060-368-01, view more51060-369-01, 51060-370-01, 51060-371-01, 51060-372-01, 51060-373-01
- Packager: Tarte, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Shake before use.
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- –
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- –
- Wear long-sleeved shirts, pants, hats and sunglasses
- Other information
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Fair
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Light
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Medium
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Medium-Tan
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Tan
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Tan-Deep
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Deep
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Rich
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - Mahogany
-
INGREDIENTS AND APPEARANCE
BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 FAIR
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-373 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 45.5 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) MICA (UNII: V8A1AW0880) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) BENZIMIDAZOLE (UNII: E24GX49LD8) DIAMOND (UNII: 6GRV67N0U2) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-373-01 1 in 1 CARTON 05/01/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 05/01/2023 BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 LIGHT
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-365 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 45.5 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) BENZIMIDAZOLE (UNII: E24GX49LD8) DIAMOND (UNII: 6GRV67N0U2) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-365-01 1 in 1 CARTON 05/01/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 05/01/2023 BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 MEDIUM
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-366 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 45.5 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) MICA (UNII: V8A1AW0880) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) DIAMOND (UNII: 6GRV67N0U2) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-366-01 1 in 1 CARTON 05/01/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 05/01/2023 BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 MEDIUM-TAN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-367 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 45.5 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) MICA (UNII: V8A1AW0880) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) DIAMOND (UNII: 6GRV67N0U2) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-367-01 1 in 1 CARTON 05/01/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 05/01/2023 BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 TAN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-368 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 45.5 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CYCLOMETHICONE 6 (UNII: XHK3U310BA) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) DIAMOND (UNII: 6GRV67N0U2) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-368-01 1 in 1 CARTON 05/01/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 05/01/2023 BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 TAN DEEP
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-369 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 45.5 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) MICA (UNII: V8A1AW0880) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) DIAMOND (UNII: 6GRV67N0U2) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-369-01 1 in 1 CARTON 05/01/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 05/01/2023 BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 DEEP
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-370 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 45.5 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIAMOND (UNII: 6GRV67N0U2) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-370-01 1 in 1 CARTON 05/01/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 05/01/2023 BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 RICH
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-371 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 45.5 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CYCLOMETHICONE 6 (UNII: XHK3U310BA) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) DIAMOND (UNII: 6GRV67N0U2) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-371-01 1 in 1 CARTON 05/01/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 05/01/2023 BB BLUR TINTED MOISTURIZER BROAD SPECTRUM SPF 30 MAHOGANY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-372 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 45.5 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) HEXYL LAURATE (UNII: 4CG9F9W01Q) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) MICA (UNII: V8A1AW0880) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) DIAMOND (UNII: 6GRV67N0U2) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-372-01 1 in 1 CARTON 05/01/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 05/01/2023 Labeler - Tarte, Inc. (027905186)