Label: DONNATAL EXTENTABS- phenobarbital, hyoscyamine sulfate, atropine sulfate, scopolamine hydrobromide tablet, film coated, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 66213-421-10, 66213-421-50 - Packager: PBM Pharmaceuticals, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 17, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Patient Insert
-
Description:
Each Donnatal Extentabs® tablet contains:
Phenobarbital, USP (3/4 gr.) 48.6 mg
Hyoscyamine Sulfate, USP 0.3111 mg
Atropine Sulfate, USP 0.0582 mg
Scopolamine Hydrobromide, USP .0.0195 mg
Each Donnatal Extentabs® tablet contains the equivalent of three Donnatal® tablets. Extentabs are designed to release the ingredi¬ents gradually to provide effects for up to twelve (12) hours.
In addition, each tablet contains the following inactive ingredients: Anhydrous Lactose, Calcium Sulfate Granular, Colloidal Silicon Dioxide, Dibasic Calcium Phosphate, Lactose Monohydrate, Magnesium Stearate, and Stearic Acid. Film Coating and Polishing Solution contains: D and C Yellow #10 Aluminum Lake, FD and C Blue #1 Aluminum Lake, Hydroxypropyl Methylcellulose, Polydextrose, Polyethylene Glycol, Titanium Dioxide, and Triacetin. The printing ink contains Titanium Dioxide. - ACTIONS:
-
INDICATIONS
Based on a review of this drug by the National Academy of Sciences - National Research Council and/or other information, FDA has classified the following indications as “possibly” effective:
For use as adjunctive therapy in the treatment of irritable bowel syndrome (irritable colon, spastic colon, mucous colitis) and acute enterocolitis.
May also be useful as adjunctive therapy in the treatment of duodenal ulcer. IT HAS NOT BEEN SHOWN CONCLUSIVELY WHETHER ANTICHOLINERGIC/ANTISPASMODIC DRUGS AID IN THE HEALING OF A DUODENAL ULCER, DECREASE THE RATE OF RECURRENCES OR PREVENT COMPLICATIONS. -
CONTRAINDICATIONS:
Glaucoma, obstructive uropathy (for example, bladder neck obstruction due to prostatic hypertrophy); obstructive disease of the gastrointestinal tract (as in achalasia, pyloroduodenal stenosis, etc.); paralytic ileus, intestinal atony of the elderly or debilitated patient; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis especially if complicated by toxic megacolon; myasthenia gravis; hiatal hernia associated with reflux esophagitis.
Donnatal Extentabs® is contraindicated in patients with known hypersensitivity to any of the ingredients. Phenobarbital is contraindicated in acute intermittent porphyria and in those patients in whom phenobarbital produces restlessness and/or excitement. -
WARNINGS:
In the presence of a high environmental temperature, heat prostration can occur with belladonna alkaloids (fever and heatstroke due to decreased sweating).
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance treatment with this drug would be inappropriate and possibly harmful.
Donnatal Extentabs® may produce drowsiness or blurred vision. The patient should be warned, should these occur, not to engage in activities requiring mental alertness, such as operating a motor vehicle or other machinery, and not to perform hazardous work.
Phenobarbital may decrease the effect of anticoagulants and necessitate larger doses of the anticoagulant for optimal effect. When phenobarbital is discontinued, the dose of the anticoagulant may have to be decreased.
Phenobarbital may be habit forming and should not be administered to individuals known to be addiction prone or to those with a history of physical and/or psychological dependence upon drugs.
Since barbiturates are metabolized in the liver, they should be used with caution and initial doses should be small in patients with hepatic dysfunction. -
PRECAUTIONS:
Use with caution in patients with: autonomic neuropathy, hepatic or renal disease, hyperthyroidism, coronary heart disease, congestive heart failure, cardiac arrhythmias, tachycardia, and hypertension.
Belladonna alkaloids may produce a delay in gastric emptying (antral stasis) which would complicate the management of gastric ulcer.
Theoretically, with overdosage, a curare-like action may occur. - Carcinogenesis, mutagenesis:
- Pregnancy Category C:
- Nursing mothers:
-
ADVERSE REACTIONS:
Adverse reactions may include xerostomia; urinary hesitancy and retention; blurred vision; tachycardia; palpitation; mydriasis; cycloplegia; increased ocular tension; loss of taste sense; headache; nervousness; drowsiness; weakness; dizziness: insomnia; nausea; vomiting; impotence; suppression of lactation; constipation; bloated feeling; musculoskeletal pain; severe allergic reaction or drug idiosyncrasies, including anaphylaxis, urticaria and other dermal manifestations; and decreased sweating. Acquired hypersensitivity to barbituates consists chiefly in allergic reactions that occur especially in persons who tend to have asthma, urticaria, angioedema and similar conditions. Hypersensitivity reactions in this category include localized swelling, particularly of the eyelids, cheeks, or lips, and erythematous dermatitis. Rarely, exfoliative dermatitis (e.g. Stevens-Johnson syndrome and toxic epidermal necrolysis) may be caused by phenobarbital and can prove fatal. The skin eruption may be associated with fever, delirium, and marked degenerative changes in the liver and other parenchymatous organs. In a few cases, megaloblastic anemia has been associated with the chronic use of phenobarbital. Elderly patients may react with symptoms of excitement, agitation, drowsiness, and other untoward manifestations to even small doses of the drug.
Phenobarbital may produce excitement in some patients, rather than a sedative effect. In patients habituated to barbiturates, abrupt withdrawal may produce delirium or convulsions. - DOSAGE AND ADMINISTRATION:
-
OVERDOSAGE:
The signs and symptoms of overdose are headache, nausea, vomiting, blurred vision, dilated pupils, hot and dry skin, dizziness, dryness of the mouth, difficulty in swallowing, and CNS stimulation. Treatment should consist of gastric lavage, emetics, and activated charcoal. If indicated, parenteral cholinergic agents such as physostigmine or bethanechol chloride should be added.
-
HOW SUPPLIED:
Donnatal Extentabs® Tablets are supplied as: film coated green, round, compressed tablets printed “P421” in black ink.
Bottles of 100 tablets
Bottles of 500 tablets
Store at 20-25°C (68-77°F) [See USP Controlled Room Temperature]. Protect from light and moisture.
Dispense in a well-closed, light-resistant container as defined in the USP using a child-resistant closure.
Also available: Donnatal® Tablets in bottles of 100 and 1000 tablets and Donnatal® Elixir in 4 fl oz bottles and 1 pint bottles. - SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL

NDC 66213-421-10 100 Tablets
Donnatal Extentabs®
Each Extentabs tablet contains:
Phenbarbital, USP . . . . 48.6 mg
Hyoscyamine Sulfate, USP . . . . . . . 0.3111 mg
Atropine Sulfate, USP . . . . . . 0.0582 mg
Scopolamine Hydrobromide, USP . . . . . . . . . 0.0195 mg
RX only
Usual Adult Usage:
See accompanying product literature for complete information.
U421-10-3
PBM Pharmaceuticals, Inc.
Charlottesville, VA 22902
Exp. Date:
Control No:
Store at 20-25°C (68-77°F) [See USP Controlled Room Temperature].
Dispense in a well closed, light resistant container as defined in the USP using a child-resistant closure. -
PRINCIPAL DISPLAY PANEL
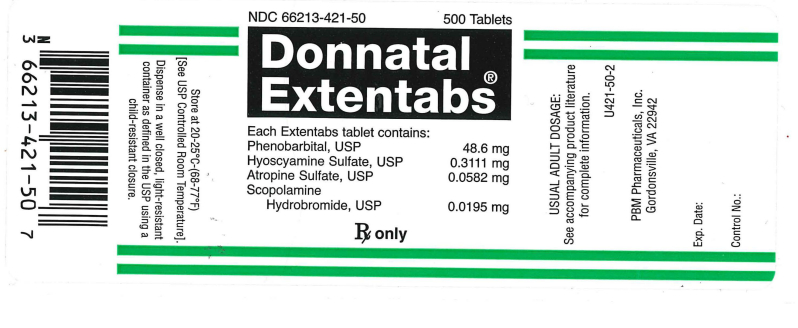
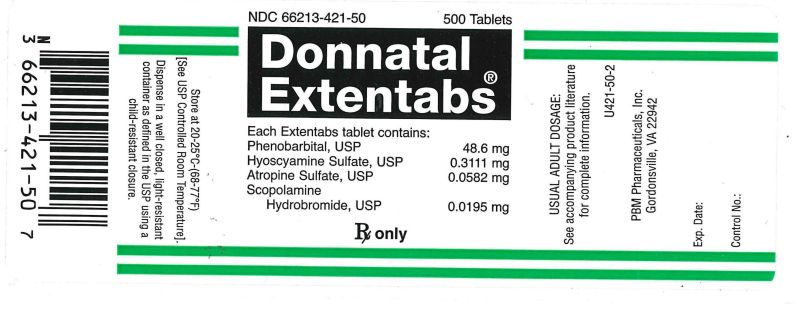
NDC 66213-421-50 500 Tablets
Donnatal Extentabs®
Each Extentabs tablet contains:
Phenbarbital, USP . . . . 48.6 mg
Hyoscyamine Sulfate, USP . . . . . . . 0.3111 mg
Atropine Sulfate, USP . . . . . . 0.0582 mg
Scopolamine Hydrobromide, USP . . . . . . . . . 0.0195 mg
RX only
Usual Adult Usage:
See accompanying product literature for complete information.
U425-50-2
PBM Pharmaceuticals, Inc.
Charlottesville, VA 22902
Exp. Date:
Control No:
Store at 20-25°C (68-77°F) [See USP Controlled Room Temperature].
Dispense in a well closed, light resistant container as defined in the USP using a child-resistant closure.
-
INGREDIENTS AND APPEARANCE
DONNATAL EXTENTABS
phenobarbital, hyoscyamine sulfate, atropine sulfate, scopolamine hydrobromide tablet, film coated, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66213-421 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Phenobarbital (UNII: YQE403BP4D) (Phenobarbital - UNII:YQE403BP4D) Phenobarbital 48.6 mg HYOSCYAMINE SULFATE (UNII: F2R8V82B84) (HYOSCYAMINE - UNII:PX44XO846X) HYOSCYAMINE SULFATE 0.3111 mg ATROPINE SULFATE (UNII: 03J5ZE7KA5) (ATROPINE - UNII:7C0697DR9I) ATROPINE SULFATE 0.0582 mg SCOPOLAMINE HYDROBROMIDE (UNII: 451IFR0GXB) (SCOPOLAMINE - UNII:DL48G20X8X) SCOPOLAMINE HYDROBROMIDE 0.0195 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CALCIUM SULFATE, UNSPECIFIED (UNII: WAT0DDB505) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STEARIC ACID (UNII: 4ELV7Z65AP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSE 2208 (4000 MPA.S) (UNII: 39J80LT57T) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL 2000 (UNII: HAF0412YIT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color green (film coated) Score no score Shape ROUND Size 10mm Flavor Imprint Code 421 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66213-421-10 100 in 1 BOTTLE, PLASTIC 2 NDC:66213-421-50 500 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/07/2008 Labeler - PBM Pharmaceuticals, Inc (785470050) Establishment Name Address ID/FEI Business Operations West-ward Pharmaceutical Corp. 001230762 manufacture, analysis, label, recovery, relabel, repack