Label: ISOPTO ATROPINE- atropine sulfate solution
- NDC Code(s): 0065-0303-55
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 7, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ISOPTO® ATROPINE safely and effectively. See full prescribing information for ISOPTO® ATROPINE
ISOPTO® ATROPINE (atropine sulfate ophthalmic solution) 1%, for topical ophthalmic use
Initial U.S. Approval: 1960
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution: 1% atropine sulfate (10mg/mL) (3)
CONTRAINDICATIONS
- Hypersensitivity or allergic reaction to any ingredient in the formulation (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions that have been reported are eye pain and stinging on administration, blurred vision, photophobia, superficial keratitis, decreased lacrimation, drowsiness, increased heart rate and blood pressure. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Alcon Laboratories, Inc., at 1-800-757-9195 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
The use of atropine and monoamine oxidase inhibitors (MAOI) is generally not recommended because of the potential to precipitate hypertensive crisis. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Mydriasis
1.2 Cycloplegia
1.3 Penalization of the healthy eye in the treatment of amblyopia
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Photophobia and Blurred Vision
5.2 Elevation of Blood Pressure
5.3 Increased Adverse Drug Reaction Susceptibility with Certain Central Nervous System Conditions
6 ADVERSE REACTIONS
6.1 Ocular Adverse Reactions
6.2 Systemic Adverse Reactions
7 DRUG INTERACTIONS
7.1 Monoamine Oxidase Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Photophobia and Blurred Vision
Photophobia and blurred vision due to pupil unresponsiveness and cycloplegia may last up to 2 weeks.
5.2 Elevation of Blood Pressure
Elevation in blood pressure from systemic absorption has been reported following conjunctival instillation of recommended doses of atropine sulfate ophthalmic solution, 1%.
5.3 Increased Adverse Drug Reaction Susceptibility with Certain Central Nervous System Conditions
Individuals with Down syndrome, spastic paralysis, or brain damage are particularly susceptible to central nervous system disturbances, cardiopulmonary, and gastrointestinal toxicity from systemic absorption of atropine.
-
6 ADVERSE REACTIONS
The following adverse reactions are described below and elsewhere in the labeling:
- Photophobia and Blurred Vision [see Warnings and Precautions (5.1)]
- Elevation in Blood Pressure [see Warnings and Precautions (5.2)]
- Increased Adverse Drug Reaction Susceptibility with Certain Central Nervous System Conditions [see Warnings and Precautions (5.3)]
The following adverse reactions have been identified following use of atropine sulfate ophthalmic solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
6.1 Ocular Adverse Reactions
Eye pain and stinging occurs upon instillation of atropine sulfate ophthalmic solution. Other commonly occurring adverse reactions include blurred vision, photophobia, superficial keratitis and decreased lacrimation. Allergic reactions such as papillary conjunctivitis, contact dermatitis, and eyelid edema may also occur less commonly.
6.2 Systemic Adverse Reactions
Systemic effects of atropine are related to its anti-muscarinic activity. Systemic adverse events reported include dryness of skin, mouth, and throat from decreased secretions from mucus membranes; drowsiness; restlessness, irritability or delirium from stimulation of the central nervous system; tachycardia; flushed skin of the face and neck.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with ISOPTO® Atropine 1% administration in pregnant women to inform a drug-associated risk. Adequate animal development and reproduction studies have not been conducted with atropine sulfate. In humans, 1% atropine sulfate is systemically bioavailable following topical ocular administration [see Clinical Pharmacology (12.3)]. ISOPTO® Atropine 1% should only be used during pregnancy if the potential benefit justifies the potential risk to the fetus.8.2 Lactation
There is no information to inform risk regarding the presence of atropine in human milk following ocular administration of ISOPTO® Atropine 1% to the mother. The effects on breastfed infants and the effects on milk production are also unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ISOPTO® Atropine 1% and any potential adverse effects on the breastfed child from ISOPTO® Atropine 1%.
8.4 Pediatric Use
Due to the potential for systemic absorption of atropine sulfate ophthalmic solution the use of ISOPTO® Atropine 1% in children under the age of 3 months is not recommended and the use in children under 3 years of age should be limited to no more than one drop per eye per day. Safety and efficacy in children above the age of 3 months has been established in adequate and well controlled trials.
-
10 OVERDOSAGE
In the event of accidental ingestion or toxic overdosage with atropine sulfate ophthalmic solution supportive care may include a short acting barbiturate or diazepam as needed to control marked excitement and convulsions. Large doses for sedation should be avoided because central depressant action may coincide with the depression occurring late in atropine poisoning. Central stimulants are not recommended.
Physostigmine, given by slow intravenous injection of 1 to 4 mg (0.5 to 1 mg in pediatric populations), rapidly abolishes delirium and coma caused by large doses of atropine. Since physostigmine is rapidly destroyed, the patient may again lapse into coma after one to two hours, and repeated doses may be required.
Artificial respiration with oxygen may be necessary. Cooling measures may be needed to help to reduce fever, especially in pediatric populations.
The fatal pediatric and adult doses of atropine are not known.
-
11 DESCRIPTION
ISOPTO® Atropine 1% is a sterile topical ophthalmic solution. Each mL of ISOPTO® Atropine 1% contains 10 mg of atropine sulfate monohydrate equivalent to 9.7 mg/mL of atropine sulfate or 8.3 mg of atropine. Atropine sulfate monohydrate is designated chemically as benzeneacetic acid, α-(hydroxymethyl)-,8-methyl-8-aza-bicyclo-[3.2.1]oct-3-yl ester, endo-(+)-, sulfate(2:1) (salt), monohydrate. Its molecular formula is (C17H23NO3)2 • H2SO4 • H2O and it is represented by the chemical structure:
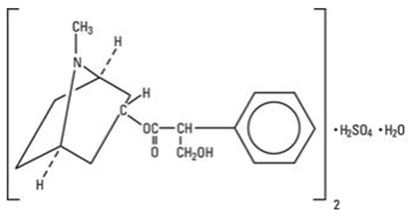
Atropine sulfate monohydrate is colorless crystals or white crystalline powder and has a molecular weight of 694.83.
ISOPTO® Atropine 1% has a pH of 3.5 to 6.0.
Active ingredient: atropine sulfate monohydrate 1.0%
Preservative: benzalkonium chloride 0.01%Inactive ingredients: hypromellose, boric acid, sodium hydroxide and/or hydrochloric acid (to adjust pH), purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Atropine acts as a competitive antagonist of the parasympathetic (and sympathetic) acetylcholine muscarinic receptors. Topical atropine on the eye induces mydriasis by inhibiting contraction of the circular pupillary sphincter muscle normally stimulated by acetylcholine. This inhibition allows the countering radial pupillary dilator muscle to contract which results in dilation of the pupil. Additionally, atropine induces cycloplegia by paralysis of the ciliary muscle which controls accommodation while viewing objects.
12.2 Pharmacodynamics
The onset of action after administration of ISOPTO Atropine 1% generally occurs in minutes with maximal effect seen in hours and the effect can last multiple days [see Clinical Studies (14)].
12.3 Pharmacokinetics
In a study of healthy subjects, after topical ocular administration of 30 µL of atropine sulfate ophthalmic solution, 1%, the mean (± SD) systemic bioavailability of l-hyoscyamine was reported to be approximately 64 ± 29% (range 19% to 95%) as compared to intravenous administration of atropine sulfate. The mean (± SD) time to maximum plasma concentration (Tmax) was approximately 28 ± 27 minutes (range 3 to 60 minutes), and the mean (±SD) peak plasma concentration (Cmax) of l-hyoscyamine was 288 ± 73 pg/mL. The mean (±SD) plasma half-life was reported to be approximately 2.5 ± 0.8 hours.
In a separate study of patients undergoing ocular surgery, after topical ocular administration of 40 µL of atropine sulfate ophthalmic solution, 1%, the mean (± SD) plasma Cmax of l‑hyoscyamine was 860 ± 402 pg/mL.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
Topical administration of ISOPTO® Atropine 1% results in mydriasis and/or cycloplegia, with efficacy demonstrated in both adults and children. The maximum effect for mydriasis is achieved in about 30–40 minutes after administration, with recovery after approximately 7–10 days. The maximum effect for cycloplegia is achieved within 60–180 minutes after administration, with recovery after approximately 7–12 days.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
- Advise patients not to drive or engage in other hazardous activities while pupils are dilated.
- Advise patient that they may experience blurry vision and sensitivity to light and should protect their eyes in bright illumination during dilation. These effects may last up to a couple weeks.
- Advise patients that they may experience drowsiness.
- Advise patients not to touch the dispenser tip to any surface, as this may contaminate the solution.
ALCON®
A Novartis company
Fort Worth, Texas 76134 USA
© 2016, 2018 Novartis -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 0065-0303-55
Alcon®
Isopto® Atropine
(atropine sulfate ophthalmic solution) 1%
5 mL Sterile
USUAL ADULT DOSAGE: One drop topically in the eye(s) up to twice daily. For other dosage information, read enclosed insert.
PRECAUTION: Do not touch dropper tip to any surface, as this may contaminate the solution.
STORAGE: Store at 2°-25°C (36°-77°F). Read enclosed insert.
Rx Only
INGREDIENTS: Each mL contains: Active: atropine sulfate monohydrate 1.0%.
Preservative: benzalkonium chloride 0.01%.
Inactives: hypromellose 0.5%, boric acid, sodium hydroxide and/or hydrochloric acid (to adjust pH), purified water.
© 2003, 2018 Novartis
Alcon®
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Product of Germany
9016388-0618
SN:
LOT:
EXP.:
GTIN: 00300650303552
NDC 0065-0303-55
Alcon®
Isopto® Atropine
(atropine sulfate ophthalmic solution) 1%
Sterile 5mL
Rx Only
INGREDIENTS: Each mL contains:
Active: atropine sulfate monohydrate 1.0%.
Preservative: benzalkonium chloride 0.01%.
Inactives: hypromellose 0.5%, boric acid, sodium hydroxide and/or hydrochloric acid (to adjust pH), purified water.
PRECAUTION: Do not touch dropper tip to any surface, as this may contaminate the solution.
USUAL ADULT DOSAGE: One drop topically in the eye(s) up to twice daily. For other dosage information, read enclosed insert. FOR TOPICAL OPHTHALMIC USE.
STORAGE: Store at 2°-25°C (36°-77°F).
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
©2018 Novartis
H15250-0318

-
INGREDIENTS AND APPEARANCE
ISOPTO ATROPINE
atropine sulfate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0065-0303 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Atropine Sulfate (UNII: 03J5ZE7KA5) (Atropine - UNII:7C0697DR9I) Atropine Sulfate 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) Hypromelloses (UNII: 3NXW29V3WO) Boric Acid (UNII: R57ZHV85D4) Sodium Hydroxide (UNII: 55X04QC32I) Hydrochloric Acid (UNII: QTT17582CB) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-0303-55 1 in 1 CARTON 08/01/2018 10/31/2023 1 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208151 08/01/2018 Labeler - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Boehringer Ingelheim Pharma GmbH & Co. KG 551147440 API MANUFACTURE(0065-0303) , ANALYSIS(0065-0303)