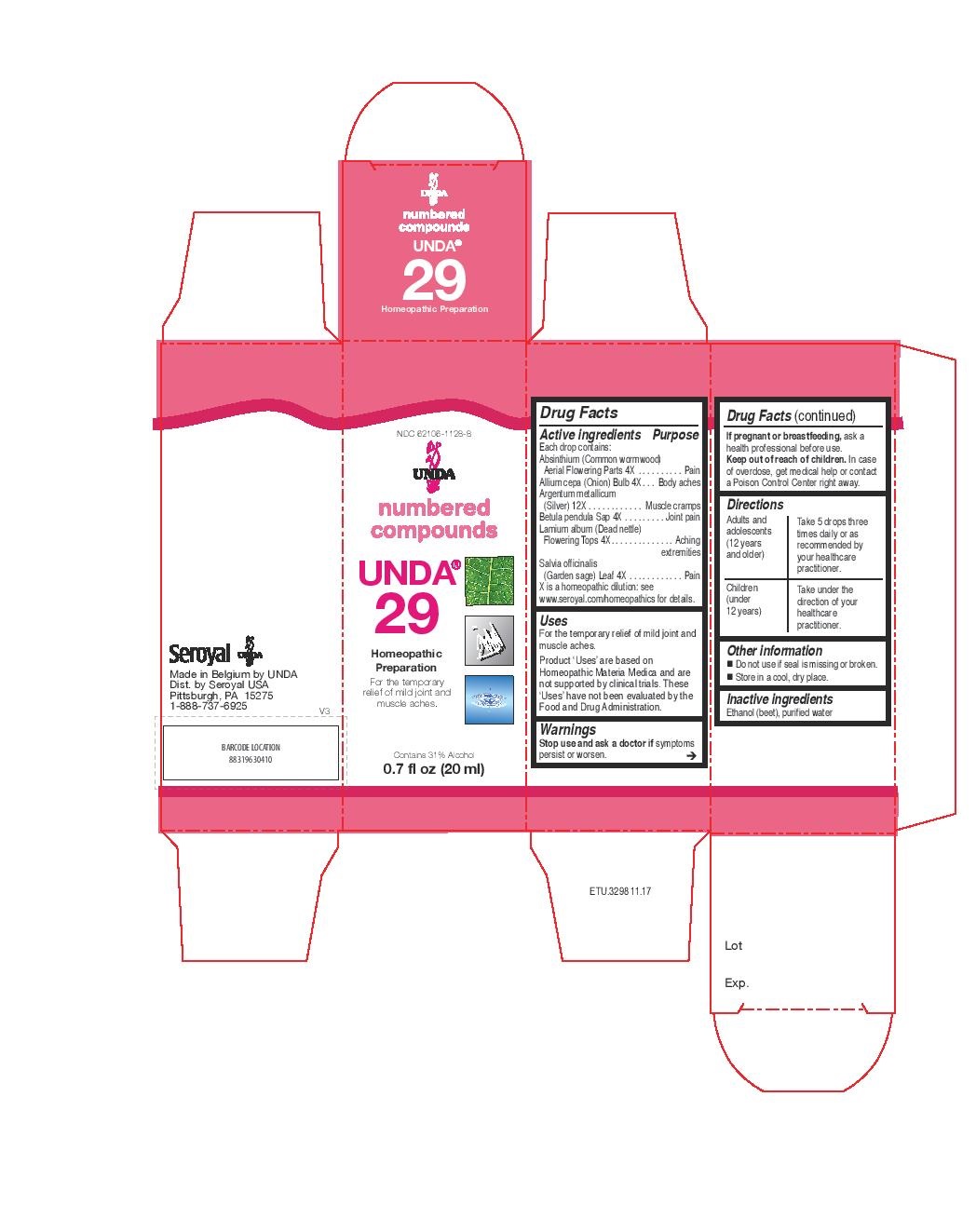
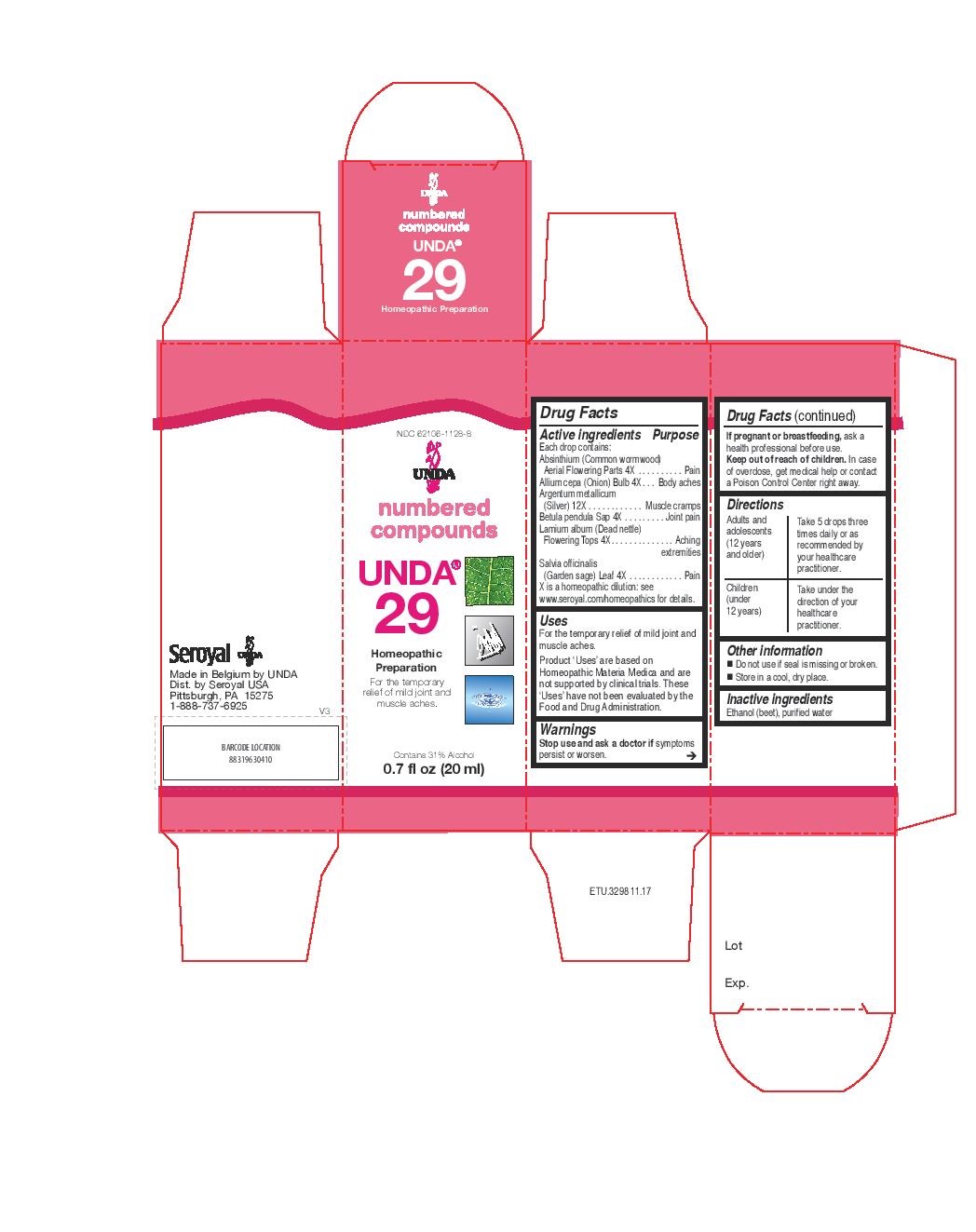
Label: UNDA 29- absinthium, allium cepa, argentum metallicum, betula pendula sap, lamium album, salvia officinalis liquid
UNDA 38- aluminium metallicum, argentum metallicum, inula helenium, sarsaparilla, stigmata maidis, valeriana officinalis liquid
- NDC Code(s): 62106-1128-8, 62106-1137-8
- Packager: Seroyal USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 9, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the relief of symptoms associated with
minor difficulties in urination.Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 29
absinthium, allium cepa, argentum metallicum, betula pendula sap, lamium album, salvia officinalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1128 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LAMIUM ALBUM (UNII: 046Y1357I6) (LAMIUM ALBUM - UNII:046Y1357I6) LAMIUM ALBUM 4 [hp_X] in 20 mL WORMWOOD (UNII: F84709P2XV) (WORMWOOD - UNII:F84709P2XV) WORMWOOD 4 [hp_X] in 20 mL ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 4 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL BETULA PENDULA WOOD (UNII: 568ZVX88G6) (BETULA PENDULA WOOD - UNII:568ZVX88G6) BETULA PENDULA WOOD 4 [hp_X] in 20 mL SAGE (UNII: 065C5D077J) (SAGE - UNII:065C5D077J) SAGE 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1128-8 1 in 1 CARTON 09/21/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/21/2015 UNDA 38
aluminium metallicum, argentum metallicum, inula helenium, sarsaparilla, stigmata maidis, valeriana officinalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1137 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM (UNII: CPD4NFA903) (ALUMINUM - UNII:CPD4NFA903) ALUMINUM 12 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL INULA HELENIUM ROOT (UNII: E55SMD6DA8) (INULA HELENIUM ROOT - UNII:E55SMD6DA8) INULA HELENIUM ROOT 4 [hp_X] in 20 mL SARSAPARILLA (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SARSAPARILLA 4 [hp_X] in 20 mL CORN SILK (UNII: 7D3VB244UX) (CORN SILK - UNII:7D3VB244UX) CORN SILK 4 [hp_X] in 20 mL VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1137-8 1 in 1 CARTON 09/21/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/21/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1128, 62106-1137)