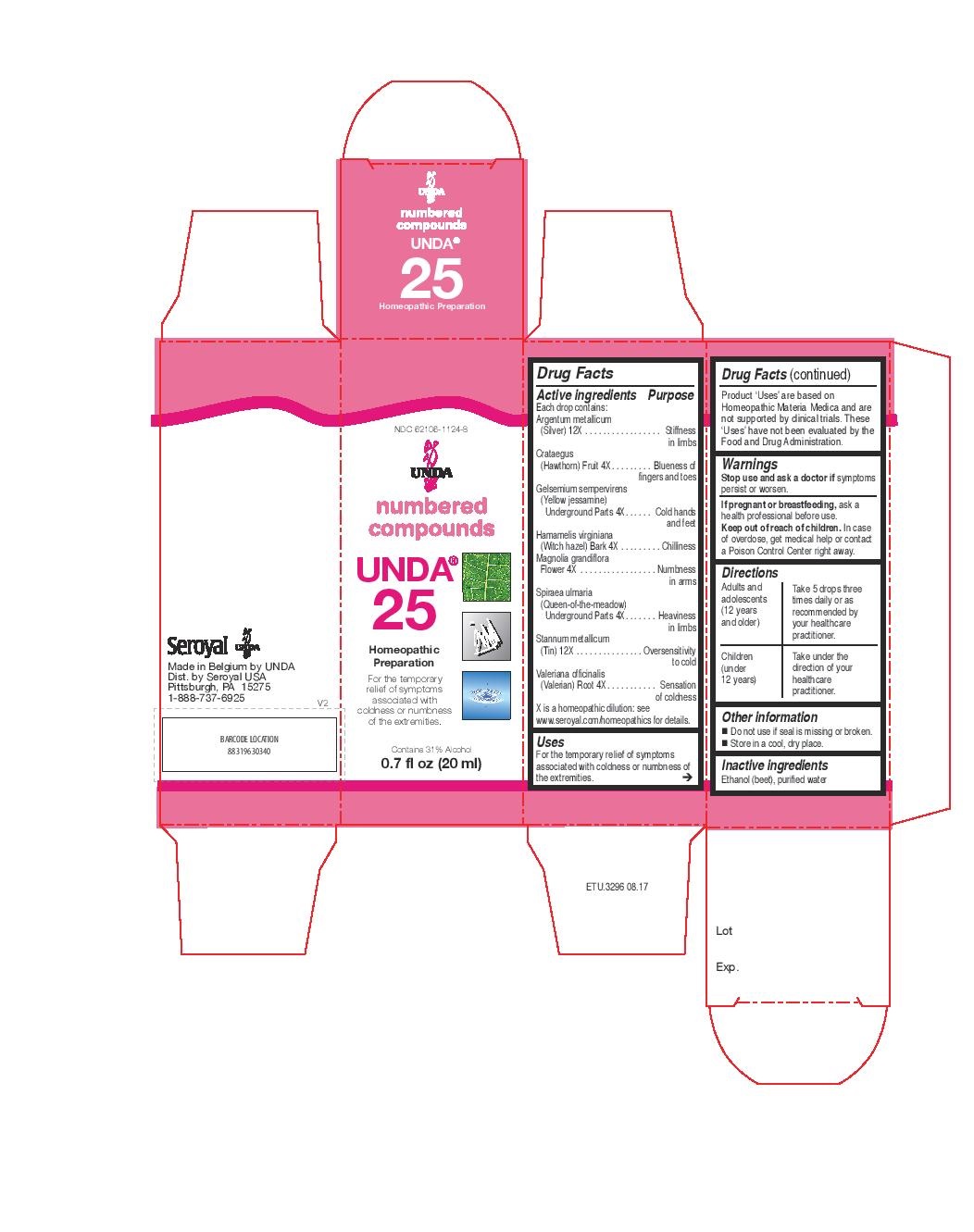
Label: UNDA 25- argentum metallicum, crataegus, gelsemium sempervirens, hamamelis virginiana, magnolia grandiflora, spiraea ulmaria, stannum metallicum, valeriana officinalis liquid
UNDA 27- antimonium crudum, borago officinalis, cetraria islandica, drosera, mentha piperita, senega officinalis liquid
UNDA 22- absinthium, aconitum napellus, argentum metallicum, boldo leaf, chamomilla, coffea tosta, jateorhiza palmata, manganum oxydatum nigrum, salvia officinalis liquid
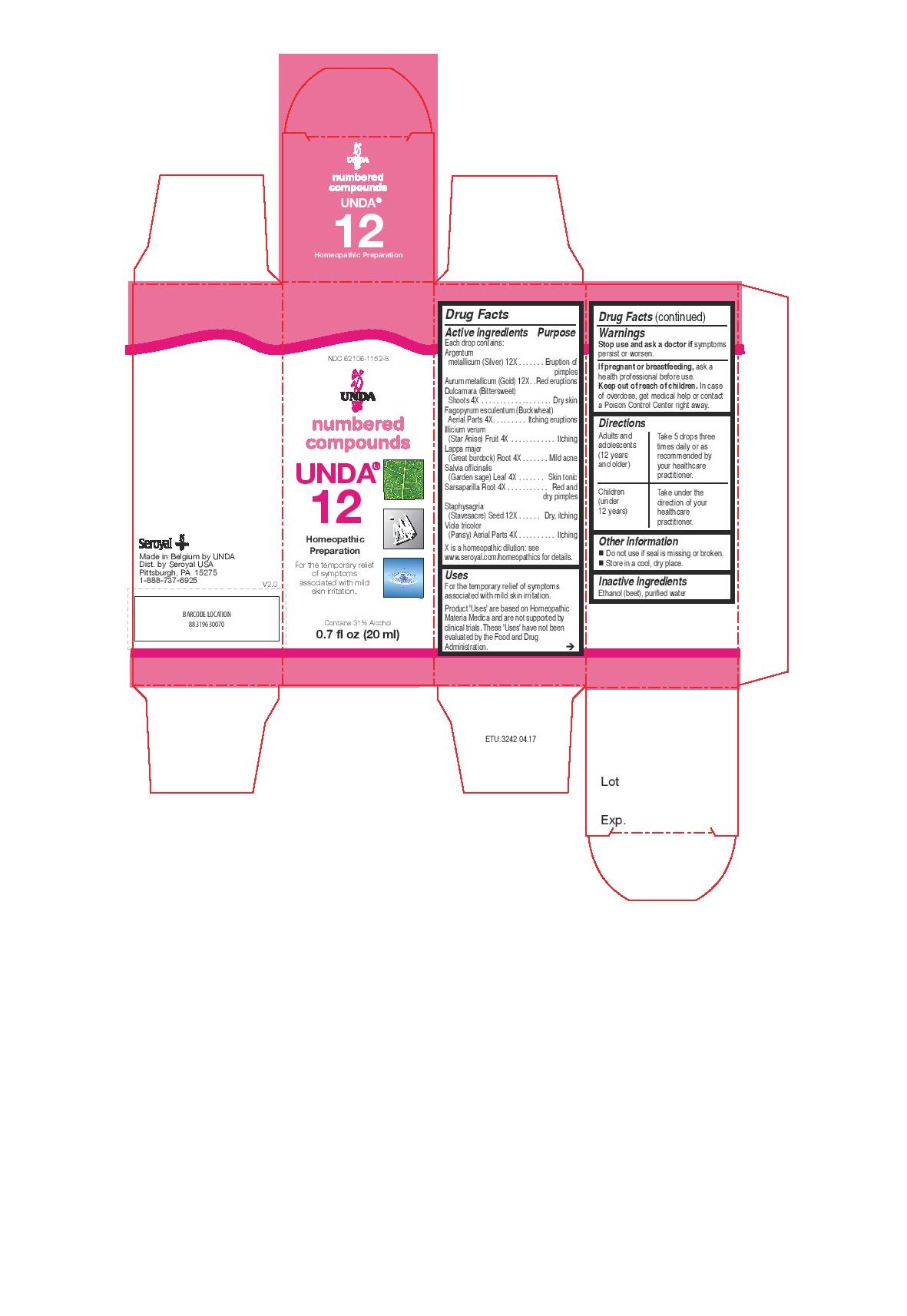
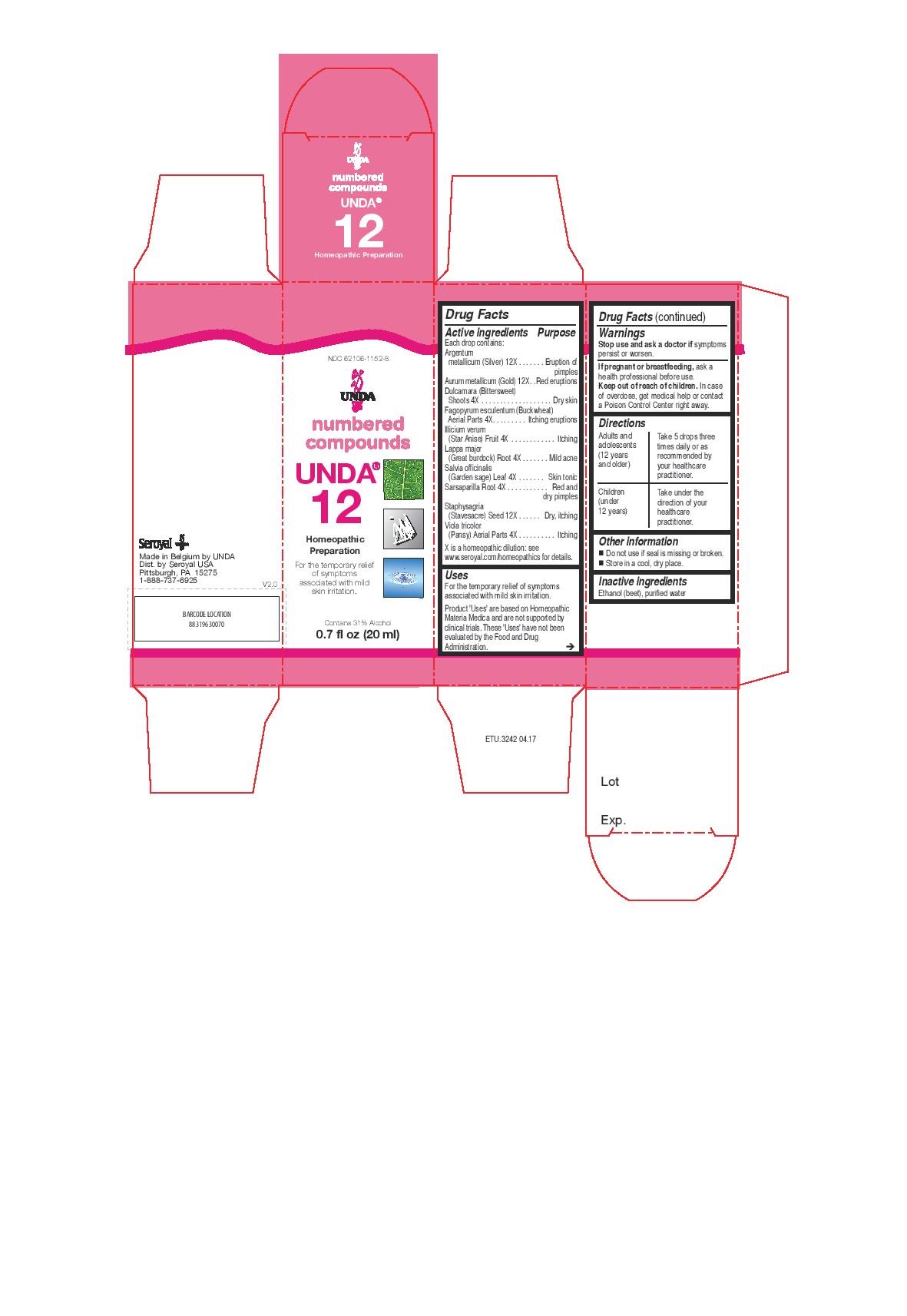
UNDA 12- argentum metallicum, aurum metallicum, dulcamara, fagopyrum esculentum, illicium verum, lappa major, salvia officinalis, sarsaparilla, staphysagria, viola tricolor liquid
- NDC Code(s): 62106-1121-8, 62106-1124-8, 62106-1126-8, 62106-1152-8
- Packager: Seroyal USA
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 15, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Argentum metallicum (Silver) 12X
Aurum metallicum (Gold) 12X
Dulcamara (Bittersweet) Shoots 4X
Fagopyrum esculentum Aerial Parts 4X
Illicium verum (Star Anise) Fruit 4X
Lappa major (Great burdock) Root 4X
Salvia officinalis (Garden sage) Leaf 4X
Sarsaparilla Root 4X
Staphysagria (Stavesacre) Seed 12X
Viola tricolor (Pansy) Aerial Parts 4X - PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with mild skin irritation.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. -
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Absinthium (Common wormwood) Aerial Parts 4X
Aconitum napellus (Aconite) Whole Plant 4X
Argentum metallicum (Silver) 12X
Boldo Leaf 4X
Chamomilla (German chamomile) Whole Flowering Plant 4X
Coffea tosta (Roasted coffee) Seed 4X
Jateorhiza palmata (Columbo) Root 4X
Manganum oxydatum nigrum (Manganese dioxide) 12X
Salvia officinalis (Garden sage) Leaf 4X - PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with mild anxiousness and nervousness.Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. -
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Argentum metallicum (Silver) 12X
Crataegus (Hawthorn) Fruit 4X
Gelsemium sempervirens (Yellow jessamine) Underground Parts 4X
Hamamelis virginiana (Witch hazel) Bark 4X
Magnolia grandiflora Flower 4X
Spiraea ulmaria (Queen-of-the-meadow) Underground Parts 4X
Stannum metallicum (Tin) 12X
Valeriana officinalis (Valerian) Root 4X - PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with
coldness or numbness of the extremities.Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. - OTHER SAFETY INFORMATION
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Ask a doctor before use if you have Persistent cough or chronic cough
such as occurs with smoking, asthma, chronic bronchitis, or emphysema and Cough that is accompanied by
excessive phlegm (mucus) or fever.Stop use and ask a doctor if symptoms persist or worsen and Cough persists for more than 1 week,
tends to recur, or is accompanied by a fever, rash, or persistent headache. A persistent cough may be a sign of a serious condition.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. - STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the relief of symptoms associated with laryngitis and voice hoarseness.Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner. - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 25
argentum metallicum, crataegus, gelsemium sempervirens, hamamelis virginiana, magnolia grandiflora, spiraea ulmaria, stannum metallicum, valeriana officinalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1124 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL FILIPENDULA ULMARIA ROOT (UNII: 997724QNDS) (FILIPENDULA ULMARIA ROOT - UNII:997724QNDS) FILIPENDULA ULMARIA ROOT 4 [hp_X] in 20 mL CRATAEGUS FRUIT (UNII: Q21UUL2105) (CRATAEGUS FRUIT - UNII:Q21UUL2105) CRATAEGUS FRUIT 4 [hp_X] in 20 mL TIN (UNII: 387GMG9FH5) (TIN - UNII:387GMG9FH5) TIN 12 [hp_X] in 20 mL VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 4 [hp_X] in 20 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 4 [hp_X] in 20 mL HAMAMELIS VIRGINIANA BARK (UNII: IH3063S9MY) (HAMAMELIS VIRGINIANA BARK - UNII:IH3063S9MY) HAMAMELIS VIRGINIANA BARK 4 [hp_X] in 20 mL MAGNOLIA GRANDIFLORA FLOWER (UNII: RV23PE6426) (MAGNOLIA GRANDIFLORA FLOWER - UNII:RV23PE6426) MAGNOLIA GRANDIFLORA FLOWER 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1124-8 1 in 1 CARTON 09/21/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/21/2015 UNDA 27
antimonium crudum, borago officinalis, cetraria islandica, drosera, mentha piperita, senega officinalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1126 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 12 [hp_X] in 20 mL BORAGE (UNII: PB618V0K2W) (BORAGE - UNII:PB618V0K2W) BORAGE 4 [hp_X] in 20 mL CETRARIA ISLANDICA SUBSP. ISLANDICA (UNII: BJ7YPN79A1) (CETRARIA ISLANDICA SUBSP. ISLANDICA - UNII:BJ7YPN79A1) CETRARIA ISLANDICA SUBSP. ISLANDICA 4 [hp_X] in 20 mL DROSERA ROTUNDIFOLIA FLOWERING TOP (UNII: 75O014T1HG) (DROSERA ROTUNDIFOLIA FLOWERING TOP - UNII:75O014T1HG) DROSERA ROTUNDIFOLIA FLOWERING TOP 4 [hp_X] in 20 mL MENTHA PIPERITA (UNII: 79M2M2UDA9) (MENTHA PIPERITA - UNII:79M2M2UDA9) MENTHA PIPERITA 4 [hp_X] in 20 mL POLYGALA SENEGA ROOT (UNII: M7T6H7D4IF) (POLYGALA SENEGA ROOT - UNII:M7T6H7D4IF) POLYGALA SENEGA ROOT 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1126-8 1 in 1 CARTON 09/21/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/21/2015 UNDA 22
absinthium, aconitum napellus, argentum metallicum, boldo leaf, chamomilla, coffea tosta, jateorhiza palmata, manganum oxydatum nigrum, salvia officinalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1121 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANGANESE DIOXIDE (UNII: TF219GU161) (MANGANESE DIOXIDE - UNII:TF219GU161) MANGANESE DIOXIDE 12 [hp_X] in 20 mL SAGE (UNII: 065C5D077J) (SAGE - UNII:065C5D077J) SAGE 4 [hp_X] in 20 mL PEUMUS BOLDUS LEAF (UNII: Q4EWM09M3O) (PEUMUS BOLDUS LEAF - UNII:Q4EWM09M3O) PEUMUS BOLDUS LEAF 4 [hp_X] in 20 mL MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 4 [hp_X] in 20 mL JATEORHIZA CALUMBA ROOT (UNII: V36I2B8LD5) (JATEORHIZA CALUMBA ROOT - UNII:V36I2B8LD5) JATEORHIZA CALUMBA ROOT 4 [hp_X] in 20 mL WORMWOOD (UNII: F84709P2XV) (WORMWOOD - UNII:F84709P2XV) WORMWOOD 4 [hp_X] in 20 mL ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 4 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL COFFEA ARABICA SEED, ROASTED (UNII: 9H58JRT35E) (COFFEA ARABICA SEED, ROASTED - UNII:9H58JRT35E) COFFEA ARABICA SEED, ROASTED 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1121-8 1 in 1 CARTON 09/21/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/21/2015 UNDA 12
argentum metallicum, aurum metallicum, dulcamara, fagopyrum esculentum, illicium verum, lappa major, salvia officinalis, sarsaparilla, staphysagria, viola tricolor liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1152 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 20 mL SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 4 [hp_X] in 20 mL FAGOPYRUM ESCULENTUM (UNII: B10M69172N) (FAGOPYRUM ESCULENTUM - UNII:B10M69172N) FAGOPYRUM ESCULENTUM 4 [hp_X] in 20 mL STAR ANISE FRUIT (UNII: CK15HA8438) (STAR ANISE FRUIT - UNII:CK15HA8438) STAR ANISE FRUIT 4 [hp_X] in 20 mL ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) (ARCTIUM LAPPA ROOT - UNII:597E9BI3Z3) ARCTIUM LAPPA ROOT 4 [hp_X] in 20 mL SAGE (UNII: 065C5D077J) (SAGE - UNII:065C5D077J) SAGE 4 [hp_X] in 20 mL SARSAPARILLA (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SARSAPARILLA 4 [hp_X] in 20 mL DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 12 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL VIOLA TRICOLOR (UNII: 9Q24RAI43V) (VIOLA TRICOLOR - UNII:9Q24RAI43V) VIOLA TRICOLOR 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1152-8 1 in 1 CARTON 09/21/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/21/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1152, 62106-1121, 62106-1124, 62106-1126)