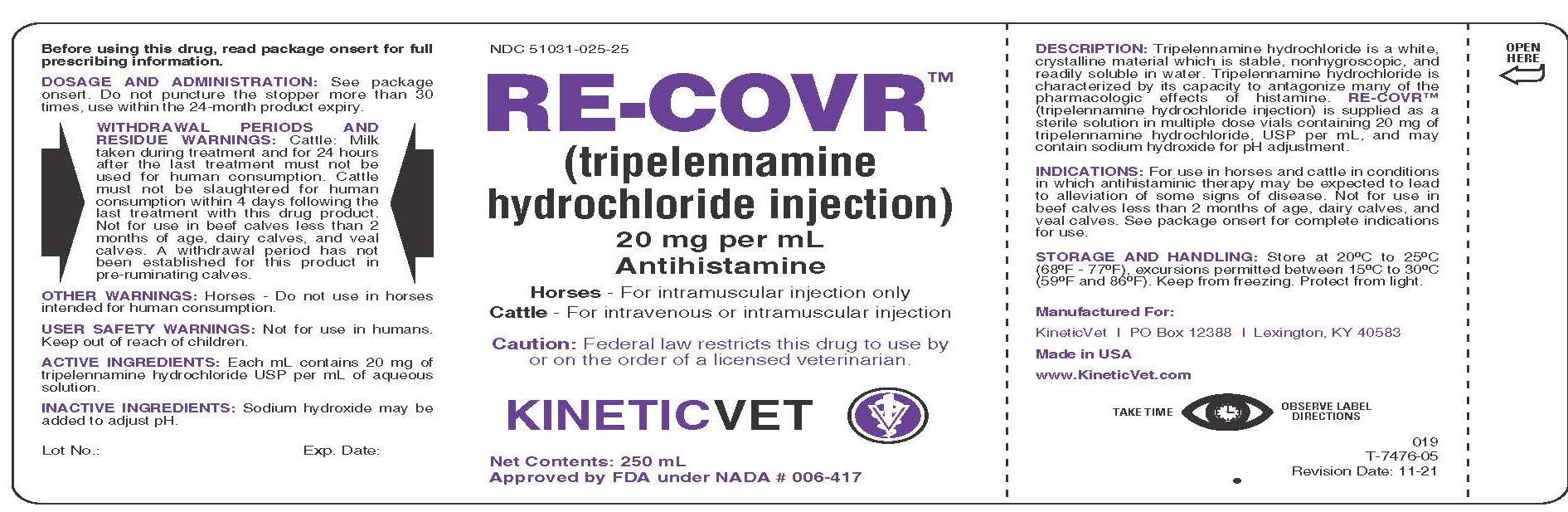
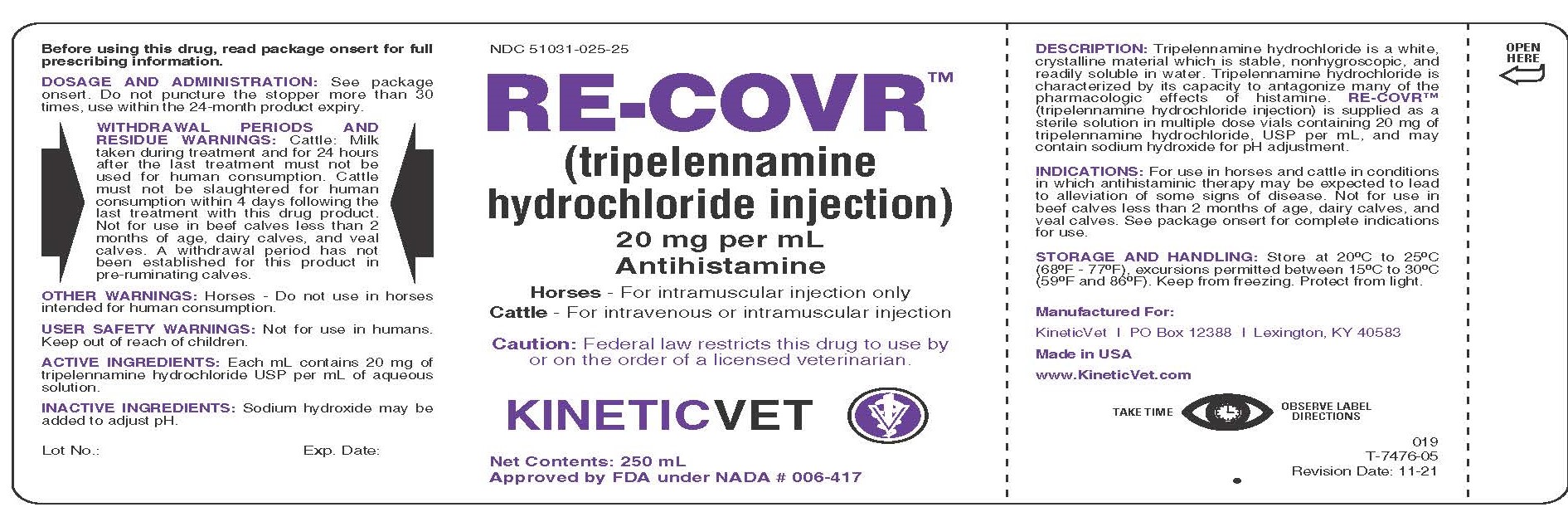
Label: RE-COVR- tripelennamine hydrochloride injection
- NDC Code(s): 51031-025-25
- Packager: Kinetic Technologies, LLC
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated February 24, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Tripelennamine hydrochloride is a white, crystalline material which is stable, nonhygroscopic, and readily soluble in water. Tripelennamine hydrochloride is characterized by its capacity to antagonize many of the pharmacologic effects of histamine. RE-COVR™ (tripelennamine hydrochloride injection) is supplied as a sterile solution in multiple dose vials containing 20 mg of tripelennamine hydrochloride, USP per mL, and may contain sodium hydroxide for pH adjustment.
- INDICATIONS:
-
DOSAGE AND ADMINISTRATION:
Horses: Administer intramuscularly only at a dose of 0.5 mg per lb of body weight (2.5 mL for each 100 lbs of body weight). This dose may be repeated in 6-12 hours if necessary.
Cattle: Administer intravenously or intramuscularly at a dose of 0.5 mg per lb of body weight (2.5 mL for each 100 lbs of body weight). This dose may be repeated in 6-12 hours if necessary. The intravenous route of administration may provide a more rapid onset of action. Use aseptic technique to administer RE-COVR™ (tripelennamine hydrochloride injection).
Warm the solution to near body temperature prior to administration. Intramuscular injection should be made into the heavy musculature of the hind leg or cervical area.
While venomous snake bites have been treated with antihistaminic drugs, other conjunctive therapy is required because of toxic reactions associated with the protein complex of venom.
Do not puncture the stopper more than 30 times, use within the 24-month product expiry.
- STORAGE AND HANDLING:
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
WITHDRAWAL PERIODS AND RESIDUE WARNINGS:
Cattle: Milk taken during treatment and for 24 hours after the last treatment must not be used for human consumption. Cattle must not be slaughtered for human consumption within 4 days following the last treatment with this drug product. Not for use in beef calves less than 2 months of age, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves.
-
ANIMAL SAFETY WARNINGS:
Administration of tripelennamine hydrochloride may give rise to excitement, ataxia, and convulsions.
Central nervous system stimulation in the form of hyperexcitability, nervousness, and muscle tremors lasting up to 20 minutes have been noted in horses following administration.
Depression of the central nervous system and incoordination may occur when the drug is used at therapeutic dose levels. Disturbances in gastrointestinal function may occur in some instances.
- OTHER WARNINGS:
- USER SAFETY WARNINGS:
- ACTIVE INGREDIENTS:
- INACTIVE INGREDIENTS:
- CONTACT INFORMATION:
- HOW SUPPLIED:
- PATIENT PACKAGE INSERT
-
PRINCIPAL DISPLAY PANEL
NDC 51031-025-25
RE-COVR™ (tripelennamine hydrochloride injection)
20 mg per mL
Antihistamine
Horses - For intramuscular injection only
Cattle - For intravenous or intramuscular injection
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
KINETICVET
Net Contents: 250 mL
Approved by FDA under NADA # 006-417
-
INGREDIENTS AND APPEARANCE
RE-COVR
tripelennamine hydrochloride injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:51031-025 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIPELENNAMINE HYDROCHLORIDE (UNII: FWV8GJ56ZN) (TRIPELENNAMINE - UNII:3C5ORO99TY) TRIPELENNAMINE 20 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51031-025-25 250 mL in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA006417 02/24/2022 Labeler - Kinetic Technologies, LLC (164935731)