Label: THE INKEY LIST SALICYLIC ACID CLEANSER- salicylic acid gel
- NDC Code(s): 81136-019-01
- Packager: Brand Evangelists for Beauty Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
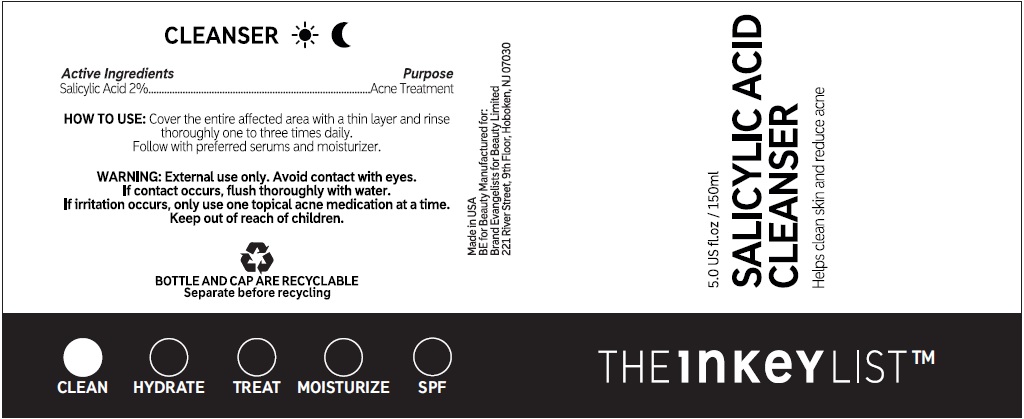
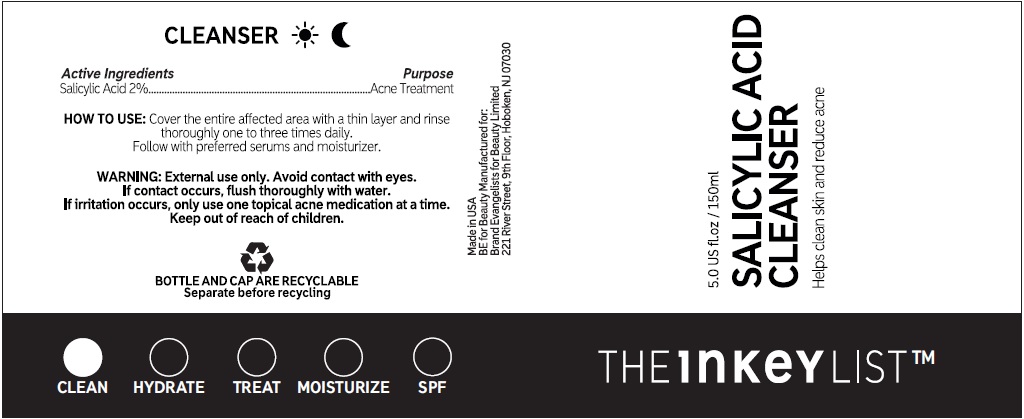
Directions
• cover the entire affected area with a thin layer and rinse thoroughly one to three times daily. • because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor. • if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Other information
-
Inactive ingredients
Water (Aqua / Eau), Propanediol, Glycerin, Sodium Methyl Cocoyl Taurate, Cocamidopropyl Betaine, PEG-120 Methyl Glucose Dioleate, PEG-150 Pentaerythrityl Tetrastearate, PEG-6 Caprylic/Capric Glycerides, Betaine, Zinc PCA, Phenoxyethanol, Sodium Chloride, Allantoin, Sodium Hydroxide, Coco-Glucoside, Glyceryl Oleate, Benzyl Alcohol, Coconut Acid, Ethylhexylglycerin, Sodium Benzoate, Citric Acid, Dehydroacetic Acid, Trisodium Ethylenediamine Disuccinate, Tocopherol, Hydrogenated Palm Glycerides Citrate.
- Questions or Comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
THE INKEY LIST SALICYLIC ACID CLEANSER
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81136-019 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROGENATED PALM GLYCERIDES CITRATE (UNII: 23AS6RA25L) WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) PEG-150 PENTAERYTHRITYL TETRASTEARATE (UNII: 8L4OOQ76AM) PEG-6 CAPRYLIC/CAPRIC GLYCERIDES (UNII: GO50W2HWO8) BETAINE (UNII: 3SCV180C9W) ZINC PIDOLATE (UNII: C32PQ86DH4) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALLANTOIN (UNII: 344S277G0Z) SODIUM HYDROXIDE (UNII: 55X04QC32I) COCO GLUCOSIDE (UNII: ICS790225B) GLYCERYL OLEATE (UNII: 4PC054V79P) BENZYL ALCOHOL (UNII: LKG8494WBH) COCONUT ACID (UNII: 40U37V505D) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM BENZOATE (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DEHYDROACETIC ACID (UNII: 2KAG279R6R) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81136-019-01 1 in 1 CARTON 05/20/2021 1 150 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 05/20/2021 Labeler - Brand Evangelists for Beauty Ltd (222990724)