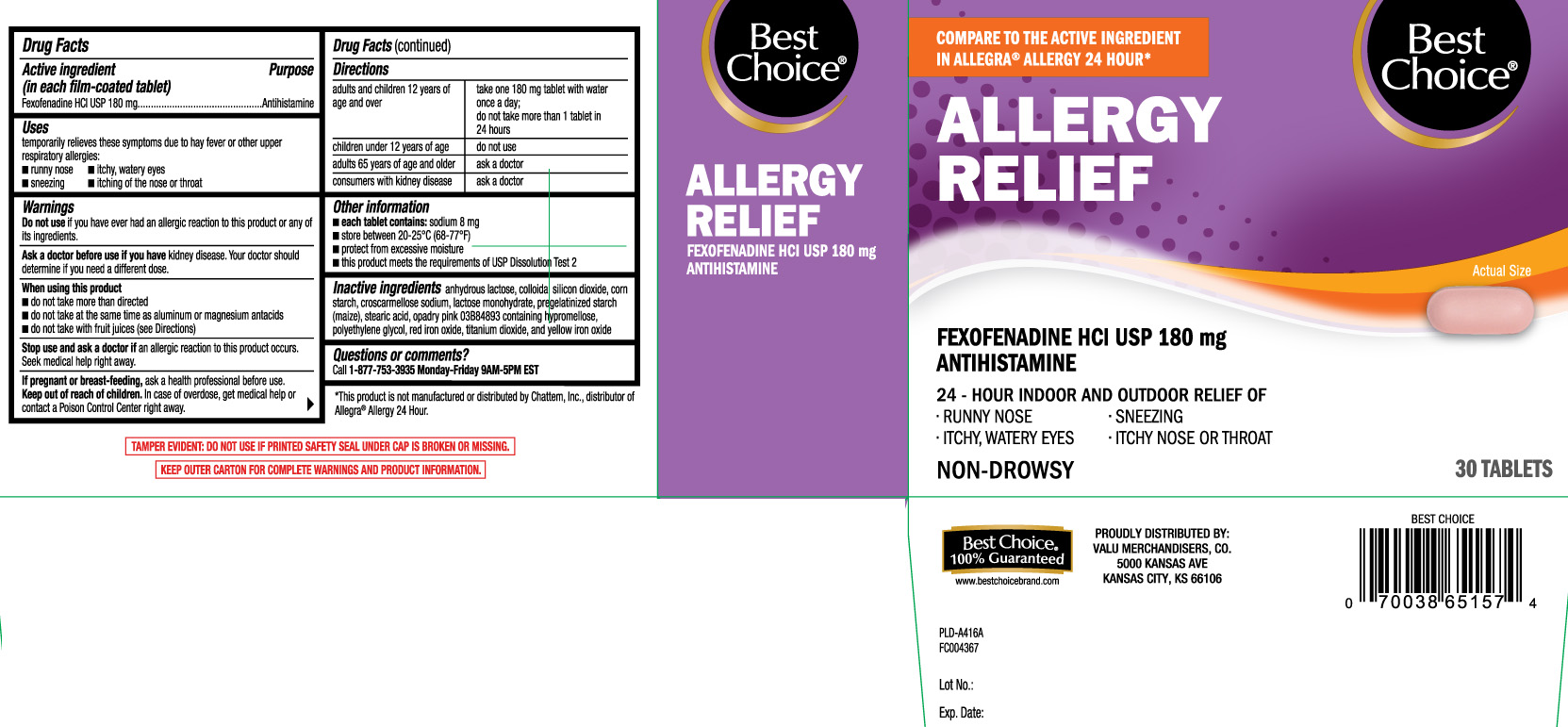
Label: ALLERGY RELIEF- fexofenadine hcl tablet
- NDC Code(s): 63941-504-30
- Packager: Best Choice (Valu Merchandisers Company)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 11, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each film-coated tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
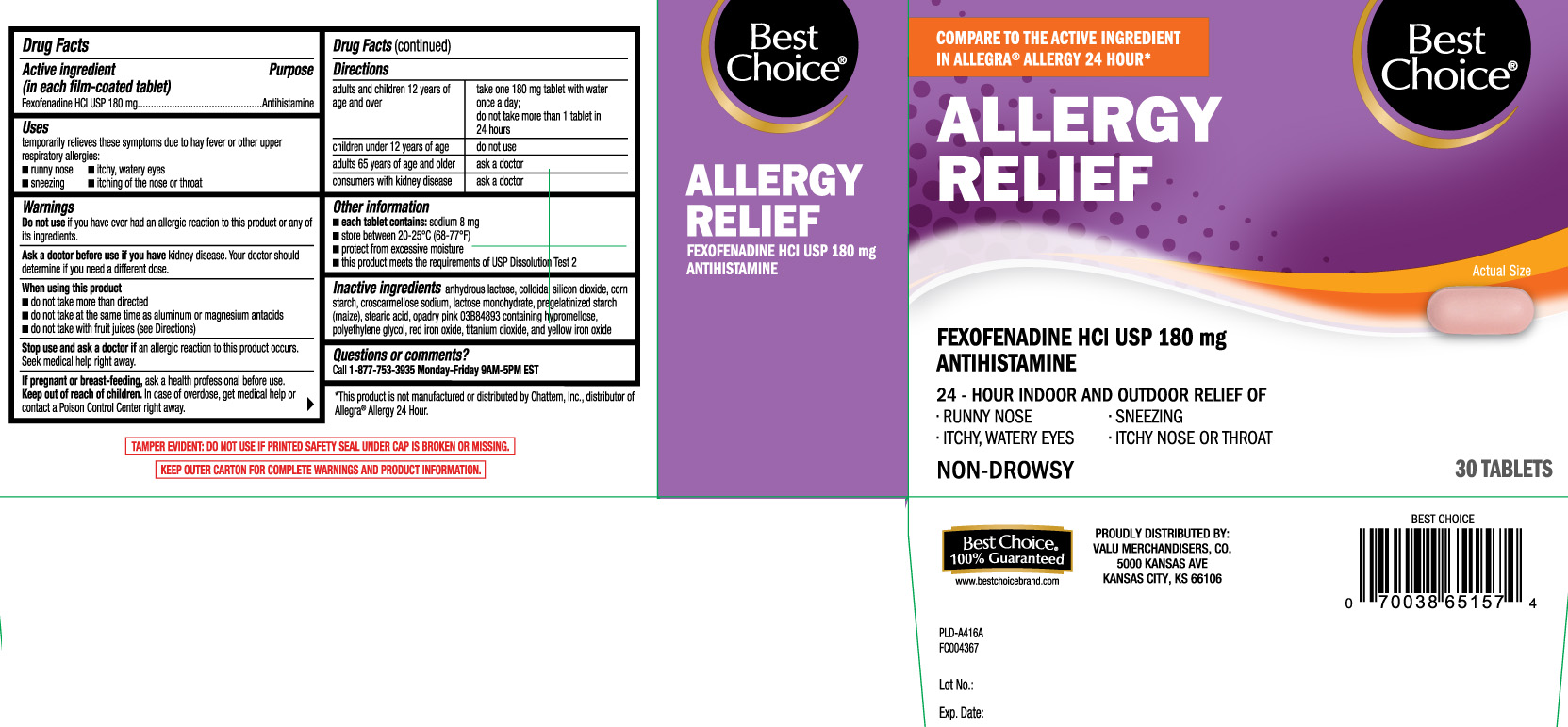
Principal Display Panel
COMPARE TO THE ACTIVE INGREDIENT IN ALLEGRA® ALLERGY 24 HOUR*
ALLERGY RELIEF
FEXOFENADINE HCI USP 180 mg
ANTIHISTAMINE
24 - HOUR INDOOR AND OUTDOOR RELIEF OF
- RUNNY NOSE
- SNEEZING
- ITCHY, WATERY EYES
- ITCHY NOSE OR THROAT
NON-DROWSY
TABLETS
†This product is not manufactured or distributed by Chattem Inc., distributor of Allegra® Allergy 24 hour
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
PROUDLY DISTRIBUTED BY:
VALU MERCHANDISERS, CO.
5000 KANSAS AVE
KANSAS CITY, KS 66106
- Product Label
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
fexofenadine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63941-504 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score no score Shape CAPSULE Size 17mm Flavor Imprint Code SG;202 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63941-504-30 1 in 1 BOX 05/31/2017 1 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079112 05/31/2017 Labeler - Best Choice (Valu Merchandisers Company) (868703513)