Label: FLUPHENAZINE DECANOATE injection, solution
- NDC Code(s): 55150-267-05
- Packager: Eugia US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
Increased Mortality in Elderly Patients with Dementia-Related Psychosis — Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Fluphenazine Decanoate Injection is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS).
-
DESCRIPTION
Fluphenazine decanoate is the decanoate ester of a trifluoromethyl phenothiazine derivative. It is a highly potent behavior modifier with a markedly extended duration of effect and has the following structural formula:
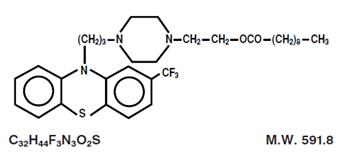
Fluphenazine Decanoate USP is a yellow solid.
Fluphenazine Decanoate Injection, USP is available as a sterile, clear, pale yellow solution for intramuscular (IM) or subcutaneous (SC) use providing 25 mg fluphenazine decanoate USP (equivalent to 18.5 mg fluphenazine) per mL in a sesame oil vehicle with 12 mg benzyl alcohol as a preservative. -
CLINICAL PHARMACOLOGY
The basic effects of fluphenazine decanoate appear to be no different from those of fluphenazine hydrochloride, with the exception of duration of action. The esterification of fluphenazine markedly prolongs the drug’s duration of effect without unduly attenuating its beneficial action.
Fluphenazine decanoate has activity at all levels of the central nervous system (CNS) as well as on multiple organ systems. The mechanism whereby its therapeutic action is exerted is unknown.
Fluphenazine differs from other phenothiazine derivatives in several respects: it is more potent on a milligram basis, it has less potentiating effect on CNS depressants and anesthetics than do some of the phenothiazines and appears to be less sedating, and it is less likely than some of the older phenothiazines to produce hypotension (nevertheless, appropriate cautions should be observed, see PRECAUTIONS and ADVERSE REACTIONS). -
INDICATIONS AND USAGE
Fluphenazine Decanoate Injection is a long-acting parenteral antipsychotic drug intended for use in the management of patients requiring prolonged parenteral neuroleptic therapy (e.g., chronic schizophrenics).
Fluphenazine Decanoate Injection has not been shown effective in the management of behavioral complications in patients with mental retardation. -
CONTRAINDICATIONS
Phenothiazines are contraindicated in patients with suspected or established subcortical brain damage.
Phenothiazine compounds should not be used in patients receiving large doses of hypnotics.
Fluphenazine Decanoate Injection is contraindicated in comatose or severely depressed states.
The presence of blood dyscrasia or liver damage precludes the use of fluphenazine decanoate.
Fluphenazine Decanoate Injection is not intended for use in children under 12 years of age.
Fluphenazine Decanoate Injection is contraindicated in patients who have shown hypersensitivity to fluphenazine; cross-sensitivity to phenothiazine derivatives may occur. -
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Fluphenazine Decanoate Injection is not approved for the treatment of patients with dementia-related psychosis (see BOXED WARNING).
Tardive Dyskinesia
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may develop in patients treated with neuroleptic (antipsychotic) drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of neuroleptic treatment, which patients are likely to develop the syndrome. Whether neuroleptic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of neuroleptic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if neuroleptic treatment is withdrawn. Neuroleptic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying disease process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, neuroleptics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic neuroleptic treatment should generally be reserved for patients who suffer from a chronic illness that, 1) is known to respond to neuroleptic drugs, and, 2) for whom alternative equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on neuroleptics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome.
(For further information about the description of tardive dyskinesia and its clinical detection, please refer to the sections on PRECAUTIONS, Information for Patients, and ADVERSE REACTIONS, Tardive Dyskinesia.)Neuroleptic Malignant Syndrome (NMS)
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary CNS pathology.
The management of NMS should include 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
Falls
Fluphenazine Decanoate Injection may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
Others
The use of this drug may impair the mental and physical abilities required for driving a car or operating heavy machinery.
Physicians should be alert to the possibility that severe adverse reactions may occur which require immediate medical attention.
Potentiation of the effects of alcohol may occur with the use of this drug.
Since there is no adequate experience in children who have received this drug, safety and efficacy in children have not been established.
Usage In Pregnancy
The safety for the use of this drug during pregnancy has not been established; therefore, the possible hazards should be weighed against the potential benefits when administering this drug to pregnant patients. -
PRECAUTIONS
General
Because of the possibility of cross-sensitivity, fluphenazine decanoate should be used cautiously in patients who have developed cholestatic jaundice, dermatoses, or other allergic reactions to phenothiazine derivatives.
Psychotic patients on large doses of a phenothiazine drug who are undergoing surgery should be watched carefully for possible hypotensive phenomena. Moreover, it should be remembered that reduced amounts of anesthetics or CNS depressants may be necessary.
The effects of atropine may be potentiated in some patients receiving fluphenazine because of added anticholinergic effects.
Fluphenazine decanoate should be used cautiously in patients exposed to extreme heat or phosphorus insecticides.
The preparation should be used with caution in patients with a history of convulsive disorders, since grand mal convulsions have been known to occur.
Use with caution in patients with special medical disorders such as mitral insufficiency or other cardiovascular disease and pheochromocytoma.
The possibility of liver damage, pigmentary retinopathy, lenticular and corneal deposits, and development of irreversible dyskinesia should be remembered when patients are on prolonged therapy.
Outside state hospitals or other psychiatric institutions, fluphenazine decanoate should be administered under the direction of a physician experienced in the clinical use of psychotropic drugs, particularly phenothiazine derivatives. Furthermore, facilities should be available for periodic checking of hepatic function, renal function, and the blood picture. Renal function of patients on long-term therapy should be monitored; if blood urea nitrogen (BUN) becomes abnormal, treatment should be discontinued.
As with any phenothiazine, the physician should be alert to the possible development of “silent pneumonias” in patients under treatment with fluphenazine decanoate.Neuroleptic drugs elevate prolactin levels; the elevation persists during chronic administration. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with a previously detected breast cancer. Although disturbances such as galactorrhea, amenorrhea, gynecomastia, and impotence have been reported, the clinical significance of elevated serum prolactin levels is unknown for most patients. An increase in mammary neoplasms has been found in rodents after chronic administration of neuroleptic drugs. Neither clinical studies nor epidemiologic studies conducted to date, however, have shown an association between chronic administration of these drugs and mammary tumorigenesis; the available evidence is considered too limited to be conclusive at this time.
Pregnancy
Non-teratogenic Effects
Neonates exposed to antipsychotic drugs, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates have required intensive care unit support and prolonged hospitalization.
Fluphenazine Decanoate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Leukopenia, Neutropenia and Agranulocytosis
In clinical trial and postmarketing experience, events of leukopenia/neutropenia and agranulocytosis have been reported temporally related to antipsychotic agents.
Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a pre-existing low WBC or a history of drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue Fluphenazine Decanoate Injection at the first sign of a decline in WBC in the absence of other causative factors.
Patients with neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1,000/mm3) should discontinue Fluphenazine Decanoate Injection and have their WBC followed until recovery.
Information for Patients
Given the likelihood that a substantial proportion of patients exposed chronically to neuroleptics will develop tardive dyskinesia, it is advised that all patients in whom chronic use is contemplated be given, if possible, full information about this risk. The decision to inform patients and/or their guardians must obviously take into account the clinical circumstances and the competency of the patient to understand the information provided. -
ADVERSE REACTIONS
Central Nervous System
The side effects most frequently reported with phenothiazine compounds are extrapyramidal symptoms including pseudoparkinsonism, dystonia, dyskinesia, akathisia, oculogyric crises, opisthotonos, and hyperreflexia. Muscle rigidity sometimes accompanied by hyperthermia has been reported following use of fluphenazine decanoate. Most often these extrapyramidal symptoms are reversible; however, they may be persistent (see below). The frequency of such reactions is related in part to chemical structure: one can expect a higher incidence with fluphenazine decanoate than with less potent piperazine derivatives or with straight-chain phenothiazines such as chlorpromazine. With any given phenothiazine derivative, the incidence and severity of such reactions depend more on individual patient sensitivity than on other factors, but dosage level and patient age are also determinants.
Extrapyramidal reactions may be alarming, and the patient should be forewarned and reassured. These reactions can usually be controlled by administration of antiparkinsonian drugs such as Benztropine Mesylate or Intravenous Caffeine and Sodium Benzoate Injection, and by subsequent reduction in dosage.
Tardive Dyskinesia
See WARNINGS. The syndrome is characterized by involuntary choreoathetoid movements which variously involve the tongue, face, mouth, lips, or jaw (e.g., protrusion of the tongue, puffing of cheeks, puckering of the mouth, chewing movements), trunk and extremities. The severity of the syndrome and the degree of impairment produced vary widely.
The syndrome may become clinically recognizable either during treatment, upon dosage reduction, or upon withdrawal of treatment. Early detection of tardive dyskinesia is important. To increase the likelihood of detecting the syndrome at the earliest possible time, the dosage of the neuroleptic drug should be reduced periodically (if clinically possible) and the patient observed for signs of the disorder. This maneuver is critical, since neuroleptic drugs may mask the signs of the syndrome.
Other CNS Effects
Occurrences of neuroleptic malignant syndrome (NMS) have been reported in patients on neuroleptic therapy (see WARNINGS, Neuroleptic Malignant Syndrome); leukocytosis, elevated CPK, liver function abnormalities, and acute renal failure may also occur with NMS.
Drowsiness or lethargy, if they occur, may necessitate a reduction in dosage; the induction of a catatonic-like state has been known to occur with dosages of fluphenazine far in excess of the recommended amounts. As with other phenothiazine compounds, reactivation or aggravation of psychotic processes may be encountered.
Phenothiazine derivatives have been known to cause, in some patients, restlessness, excitement, or bizarre dreams.
Autonomic Nervous System
Hypertension and fluctuations in blood pressure have been reported with fluphenazine.
Hypotension has rarely presented a problem with fluphenazine. However, patients with pheochromocytoma, cerebral, vascular or renal insufficiency, or a severe cardiac reserve deficiency such as mitral insufficiency appear to be particularly prone to hypotensive reactions with phenothiazine compounds, and should therefore be observed closely when the drug is administered. If severe hypotension should occur, supportive measures including the use of intravenous vasopressor drugs should be instituted immediately. Norepinephrine Bitartrate Injection is the most suitable drug for this purpose: epinephrine should not be used since phenothiazine derivatives have been found to reverse its action, resulting in a further lowering of blood pressure.
Autonomic reactions including nausea and loss of appetite, salivation, polyuria, perspiration, dry mouth, headache, and constipation may occur. Autonomic effects can usually be controlled by reducing or temporarily discontinuing dosage.
In some patients, phenothiazine derivatives have caused blurred vision, glaucoma, bladder paralysis, fecal impaction, paralytic ileus, tachycardia, or nasal congestion.
Metabolic and Endocrine
Weight change, peripheral edema, abnormal lactation, gynecomastia, menstrual irregularities, false results on pregnancy tests, impotency in men and increased libido in women have all been known to occur in some patients on phenothiazine therapy.
Allergic Reactions
Skin disorders such as itching, erythema, urticaria, seborrhea, photosensitivity, eczema and even exfoliative dermatitis have been reported with phenothiazine derivatives. The possibility of anaphylactoid reactions occurring in some patients should be borne in mind.
Hematologic
Routine blood counts are advisable during therapy since blood dyscrasias including leukopenia, agranulocytosis, thrombocytopenic or nonthrombocytopenic purpura, eosinophilia, and pancytopenia have been observed with phenothiazine derivatives. Furthermore, if any soreness of the mouth, gums, or throat, or any symptoms of upper respiratory infection occur and confirmatory leukocyte count indicates cellular depression, therapy should be discontinued and other appropriate measures instituted immediately.
Hepatic
Liver damage as manifested by cholestatic jaundice may be encountered, particularly during the first months of therapy; treatment should be discontinued if this occurs. An increase in cephalin flocculation, sometimes accompanied by alterations in other liver function tests, has been reported in patients receiving the enanthate ester of fluphenazine (a closely related compound) who have had no clinical evidence of liver damage.
Others
Sudden, unexpected and unexplained deaths have been reported in hospitalized psychotic patients receiving phenothiazines. Previous brain damage or seizures may be predisposing factors; high doses should be avoided in known seizure patients. Several patients have shown sudden flare-ups of psychotic behavior patterns shortly before death. Autopsy findings have usually revealed acute fulminating pneumonia or pneumonitis, aspiration of gastric contents, or intramyocardial lesions.
Although this is not a general feature of fluphenazine, potentiation of CNS depressants (opiates, analgesics, antihistamines, barbiturates, alcohol) may occur.
The following adverse reactions have also occurred with phenothiazine derivatives: Systemic lupus erythematosus-like syndrome, hypotension severe enough to cause fatal cardiac arrest, altered electrocardiographic and electroencephalographic tracings, altered cerebrospinal fluid proteins, cerebral edema, asthma, laryngeal edema, and angioneurotic edema; with long-term use, skin pigmentation, and lenticular and corneal opacities.
Injections of fluphenazine decanoate are extremely well tolerated, local tissue reactions occurring only rarely. -
DOSAGE AND ADMINISTRATION
Fluphenazine Decanoate Injection may be given IM or SC. A dry syringe and needle of at least 21 gauge should be used. Use of a wet needle or syringe may cause the solution to become cloudy.
To begin therapy with Fluphenazine Decanoate Injection, the following regimens are suggested:
For most patients, a dose of 12.5 to 25 mg (0.5 to 1 mL) may be given to initiate therapy. The onset of action generally appears between 24 and 72 hours after injection and the effects of the drug on psychotic symptoms becomes significant within 48 to 96 hours. Subsequent injections and the dosage interval are determined in accordance with the patient’s response. When administered as maintenance therapy, a single injection may be effective in controlling schizophrenic symptoms up to four weeks or longer. The response to a single dose has been found to last as long as six weeks in a few patients on maintenance therapy.
It may be advisable that patients who have no history of taking phenothiazines should be treated initially with a shorter-acting form of fluphenazine before administering the decanoate to determine the patient’s response to fluphenazine and to establish appropriate dosage. For psychotic patients who have been stabilized on a fixed daily dosage of Fluphenazine Hydrochloride Tablets, USP or Fluphenazine Hydrochloride Elixir, USP conversion of therapy from these short-acting oral forms to the long-acting Fluphenazine Decanoate Injection may be indicated.
Appropriate dosage of Fluphenazine Decanoate Injection should be individualized for each patient and responses carefully monitored. No precise formula can be given to convert to use of Fluphenazine Decanoate Injection; however, a controlled multicentered study,* in patients receiving oral doses from 5 to 60 mg fluphenazine hydrochloride daily, showed that 20 mg fluphenazine hydrochloride daily was equivalent to 25 mg (1 mL) of Fluphenazine Decanoate Injection every three weeks. This represents an approximate conversion ratio of 12.5 mg (0.5 mL) of decanoate every three weeks for every 10 mg of fluphenazine hydrochloride daily.
Once conversion to Fluphenazine Decanoate Injection is made, careful clinical monitoring of the patient and appropriate dosage adjustment should be made at the time of each injection.
Severely agitated patients may be treated initially with a rapid-acting phenothiazine compound such as Fluphenazine Hydrochloride Injection—see Package Insert accompanying that product for complete information. When acute symptoms have subsided, 25 mg (1 mL) of Fluphenazine Decanoate Injection may be administered; subsequent dosage is adjusted as necessary.
“Poor risk” patients (those with known hypersensitivity to phenothiazines, or with disorders that predispose to undue reactions): Therapy may be initiated cautiously with oral or parenteral fluphenazine hydrochloride (see Package Inserts accompanying these products for complete information). When the pharmacologic effects and an appropriate dosage are apparent, an equivalent dose of fluphenazine decanoate may be administered. Subsequent dosage adjustments are made in accordance with the response of the patient.
The optimal amount of the drug and the frequency of administration must be determined for each patient, since dosage requirements have been found to vary with clinical circumstances as well as with individual response to the drug.
Dosage should not exceed 100 mg. If doses greater than 50 mg are deemed necessary, the next dose and succeeding doses should be increased cautiously in increments of 12.5 mg.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. -
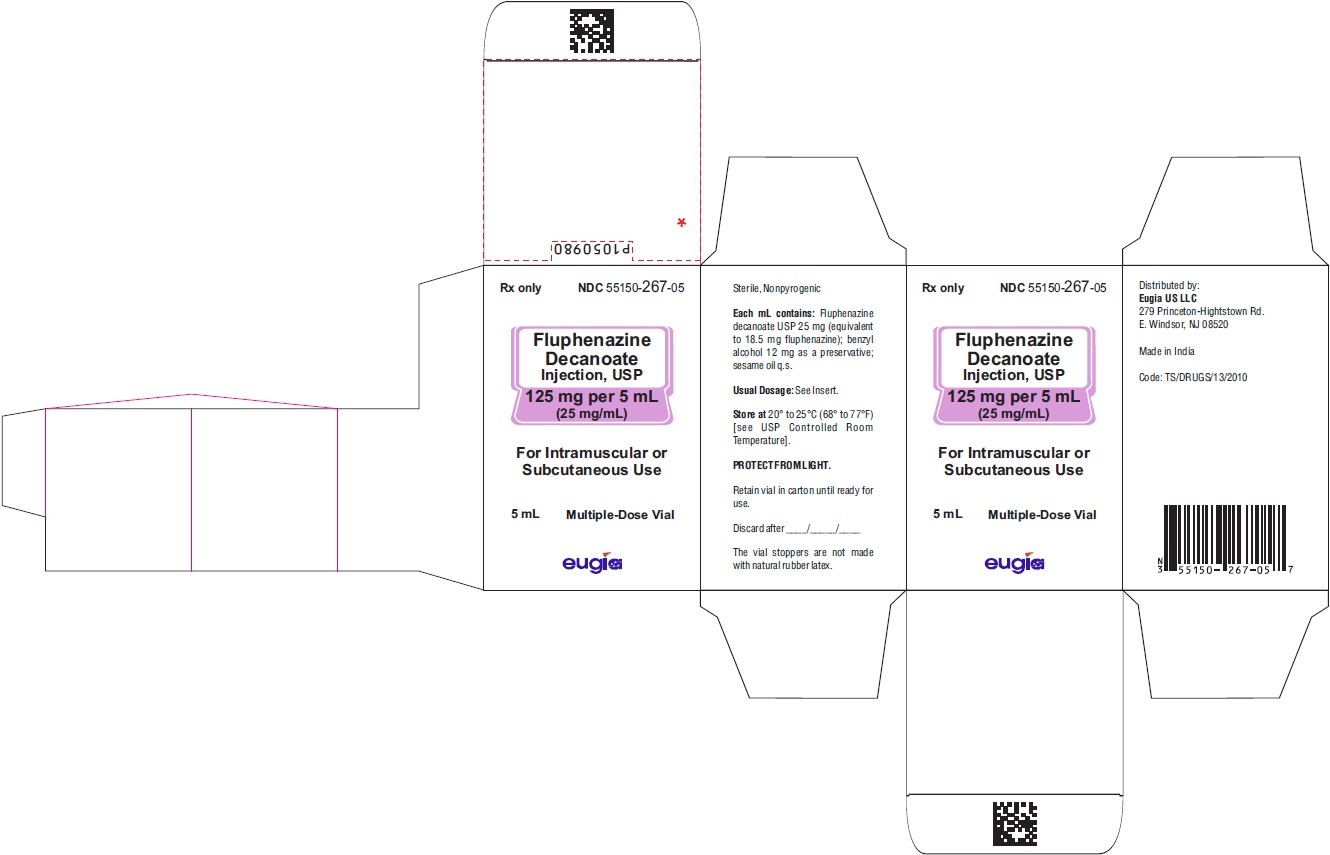
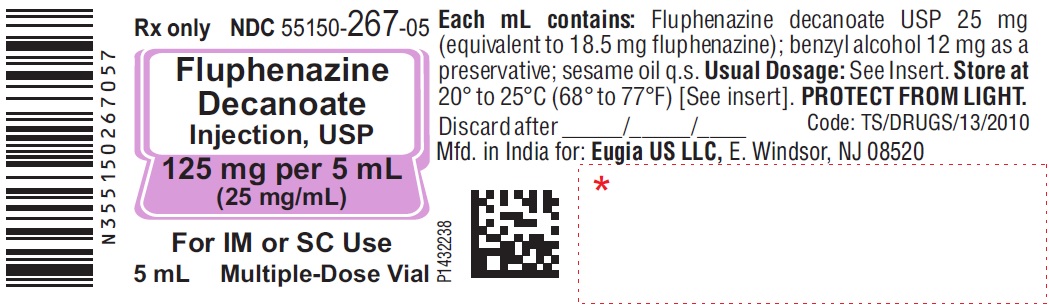
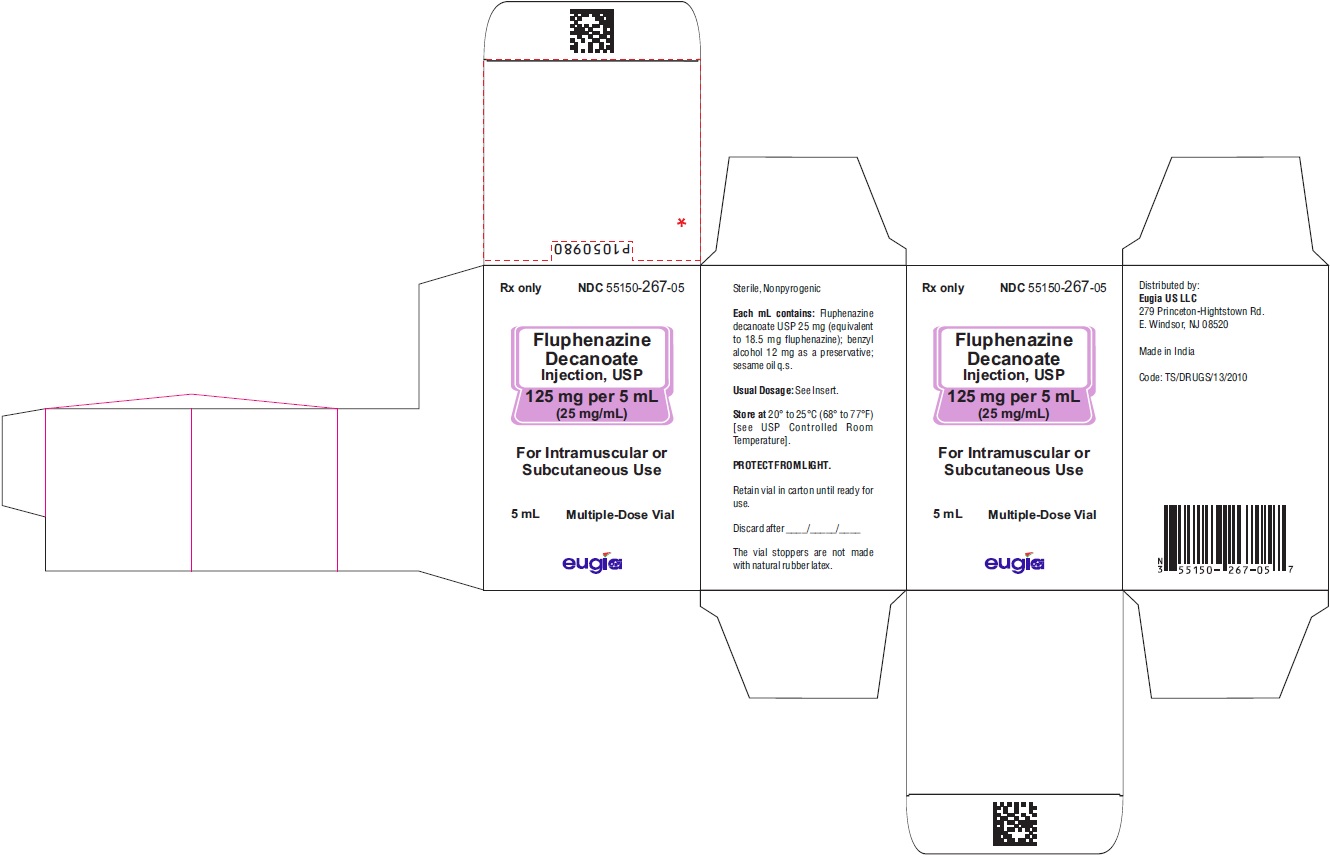
HOW SUPPLIED
Fluphenazine Decanoate Injection, USP is a sterile, clear, pale yellow solution and is supplied as follows:
125 mg per 5 mL (25 mg/mL)
5 mL Multiple-Dose Vials
Packaged Individually NDC 55150-267-05
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Protect from light (keep in outer carton).
The vial stoppers are not made with natural rubber latex.
* Schooler, N.R.: The Initiation of Long-Term Pharmacotherapy in Schizophrenia: Dosage and Side Effect Comparisons between Oral and Depot Fluphenazine Pharmakopsych. 9:159-169, 1976.
Distributed by:
Eugia US LLC
279 Princeton-Hightstown Rd.
E. Windsor, NJ 08520
Manufactured by:
Eugia Pharma Specialities Limited
Hyderabad - 500032
India
Revised: July 2023 - PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 125 mg per 5 mL (25 mg/mL) - Container Label
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 125 mg per 5 mL (25 mg/mL) - Container-Carton (1 Vial)
-
INGREDIENTS AND APPEARANCE
FLUPHENAZINE DECANOATE
fluphenazine decanoate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55150-267 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUPHENAZINE DECANOATE (UNII: FMU62K1L3C) (FLUPHENAZINE - UNII:S79426A41Z) FLUPHENAZINE DECANOATE 125 mg in 5 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) SESAME OIL (UNII: QX10HYY4QV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55150-267-05 1 in 1 CARTON 10/17/2017 1 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207739 10/17/2017 Labeler - Eugia US LLC (968961354) Establishment Name Address ID/FEI Business Operations Eugia Pharma Specialities Limited 650498244 ANALYSIS(55150-267) , MANUFACTURE(55150-267) , PACK(55150-267)