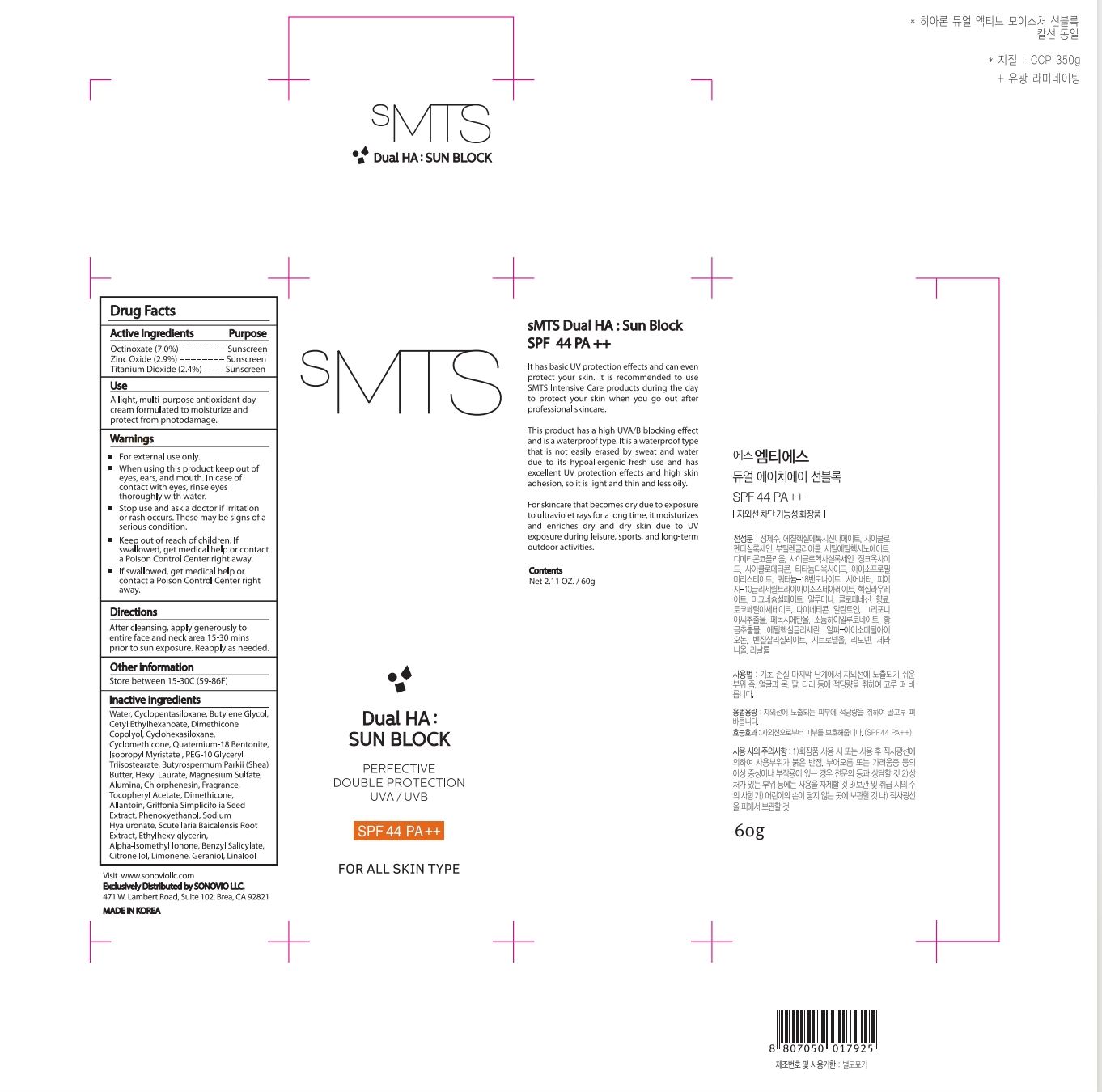
Label: SMTS DUAL HA SUN BLOCK cream
- NDC Code(s): 84609-001-01
- Packager: Sonovio LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- Uses
-
Warnings
①For external use only.
② When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
③Stop use and ask a doctor if irrrtion or rash occurs. These may be signs of a serious condition.
④Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away,
⑤If swallowed, get medical help or contact a Poison Control Center rightaway. - DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
-
Inactive Ingredients
Water, Cyclopentasiloxane, Butylene Glycol, Cetyl Ethylhexanoate, Dimethicone Copolyol, Cyclohexasiloxane,
Cyclomethicone, Quaternium-18 Bentonite, lsopropyl Myristate , PEG-10 Glyceryl Triisostearate,
Butyrospermum Parkii (Shea) Butter, Hexyl Laurate, Magnesium Sulfate, Alumina, Chlorphenesin, Fragrance,
Tocopheryl Acetate, Dimethicone, Allantoin, Griffonia Simplicifolia Seed Extract, Phenoxyethanol, Sodium
Hyaluronate, Scutellaria Baicalensis Root Extract, Ethylhexylglycerin, Alpha-lsomethyl lonone, Benzyl Salicylate,Citronellol, Limonene, Geraniol, Linalool
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SMTS DUAL HA SUN BLOCK
smts dual ha sun block creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84609-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 100 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.4 g in 100 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7 g in 100 g Inactive Ingredients Ingredient Name Strength ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) PHENOXYETHANOL (UNII: HIE492ZZ3T) LIMONENE, (+)- (UNII: GFD7C86Q1W) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) CHLORPHENESIN (UNII: I670DAL4SZ) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BENTOQUATAM (UNII: 7F465U79Q1) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) PEG-10 GLYCERYL TRIISOSTEARATE (UNII: 8U46MJI8HO) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) LINALOOL, (+/-)- (UNII: D81QY6I88E) GRIFFONIA SIMPLICIFOLIA SEED (UNII: LUS5142TMY) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DIMETHICONE (UNII: 92RU3N3Y1O) ALLANTOIN (UNII: 344S277G0Z) CYCLOMETHICONE (UNII: NMQ347994Z) ALUMINUM OXIDE (UNII: LMI26O6933) BENZYL SALICYLATE (UNII: WAO5MNK9TU) GERANIOL (UNII: L837108USY) HEXYL LAURATE (UNII: 4CG9F9W01Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84609-001-01 60 g in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/01/2024 Labeler - Sonovio LLC (119201052) Establishment Name Address ID/FEI Business Operations Sonovio LLC 119201052 manufacture(84609-001)