Label: GLYCOPYRROLATE tablet
- NDC Code(s): 49884-065-01, 49884-066-01
- Packager: Par Pharmaceutical, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 16, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Glycopyrrolate tablets contain the synthetic anticholinergic glycopyrrolate. Glycopyrrolate is a quaternary ammonium compound with the following chemical name:
3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethylpyrrolidinium bromide. Its empirical formula is C19H28BrNO3, its molecular weight is 398.33, and its structural formula is:
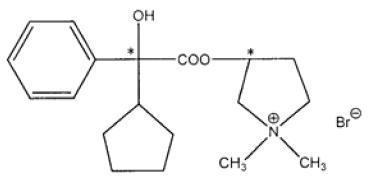
Each 1 mg tablet contains: Glycopyrrolate, USP 1mg
Each 2 mg tablet contains: Glycopyrrolate, USP 2mg
Inactive Ingredients: Dibasic Calcium Phosphate, Lactose, Magnesium Stearate, Povidone, Sodium Starch Glycolate
-
CLINICAL PHARMACOLOGY
Glycopyrrolate, like other anticholinergic (antimuscarinic) agents, inhibits the action of acetylcholine on structures innervated by postganglionic cholinergic nerves and on smooth muscles that respond to acetylcholine but lack cholinergic innervation. These peripheral cholinergic receptors are present in the autonomic effector cells of smooth muscle, cardiac muscle, the sino-atrial node, the atrioventricular node, exocrine glands, and, to a limited degree, in the autonomic ganglia. Thus, it diminishes the volume and free acidity of gastric secretions and controls excessive pharyngeal, tracheal, and bronchial secretions.
Glycopyrrolate antagonizes muscarinic symptoms (e.g., bronchorrhea, bronchospasm, bradycardia, and intestinal hypermotility) induced by cholinergic drugs such as the anticholinesterases.
The highly polar quaternary ammonium group of glycopyrrolate limits its passage across lipid membranes, such as the blood-brain barrier, in contrast to atropine sulfate and scopolamine hydrobromide, which are non-polar tertiary amines which penetrate lipid barriers easily.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Glaucoma; obstructive uropathy (for example, bladder neck obstruction due to prostatic hypertrophy); obstructive disease of the gastrointestinal tract (as in achalasia, pyloroduodenal stenosis, etc.); paralytic ileus; intestinal atony of the elderly or debilitated patient; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis; toxic megacolon complicating ulcerative colitis; myasthenia gravis. Glycopyrrolate tablets are contraindicated in those patients with a hypersensitivity to glycopyrrolate.
-
WARNINGS
In the presence of a high environmental temperature, heat prostration (fever and heat stroke due to decreased sweating) can occur with use of glycopyrrolate.
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomyor colostomy. In this instance treatment with this drug would be inappropriate and possibly harmful.
Glycopyrrolate may produce drowsiness and blurred vision. In this event, the patient should be warned not to engage in activities requiring mental alertness such as operating a motor vehicle or other machinery, or performing hazardous work while taking this drug.
Theoretically, with overdosage, a curare-like action may occur, i.e., neuro-muscular blockade leading to muscular weakness and possible paralysis.
Pregnancy
The safety of this drug during pregnancy has not been established. The use of any drug during pregnancy requires that potential benefits of the drug be weighed against possible hazards to mother and child. Reproduction studies in rats revealed no teratogenic effects from glycopyrrolate; however, the potent anticholinergic action of this agent resulted in diminished rates of conception and of survival at weaning, in a dose-related manner. Other studies in dogs suggest that this may be due to diminished seminal secretion which is evident at high doses of glycopyrrolate. Information on possible adverse effects in the pregnant female is limited to uncontrolled data derived from marketing experience. Such experience has revealed no reports of teratogenic or other fetus-damaging potential. No controlled studies to establish the safety of the drug in pregnancy have been performed.
-
PRECAUTIONS
Use glycopyrrolate with caution in the elderly and in all patients with:
- Autonomic neuropathy.
- Hepatic or renal disease.
- Ulcerative colitis-large doses may suppress intestinal motility to the point of producing a paralytic ileus and for this reason may precipitate or aggravate “toxic megacolon,” a serious complication of the disease.
- Hyperthyrodism, coronary heart disease, congestive heart failure, cardiac tachyarrhythmias, tachycardia, hypertension and prostatic hypertrophy.
- Hiatal hernia associated with reflux esophagitis, since anticholinergic drugs may aggravate this condition.
-
ADVERSE REACTIONS
Anticholinergics produce certain effects, most of which are extensions of their fundamental pharmacological actions. Adverse reactions to anticholinergics in general may include xerostomia; decreased sweating; urinary hesitancy and retention; blurred vision; tachycardia; palpitations; dilatation of the pupil; cycloplegia; increased ocular tension; loss of taste; headaches; nervousness; mental confusion; drowsiness; weakness; dizziness; insomnia; nausea; vomiting; constipation; bloated feeling; impotence; suppression of lactation; severe allergic reaction or drug idiosyncrasies including anaphylaxis, urticaria and other dermal manifestations.
Glycopyrrolate is chemically a quaternary ammonium compound; hence, its passage across lipid membranes, such as the blood-brain barrier, is limited in contrast to atropine sulfate and scopolamine hydrobromide. For this reason the occurrence of CNS related side effects is lower, in comparison to their incidence following administration of anticholinergics which are chemically tertiary amines that can cross this barrier readily.
-
OVERDOSAGE
The symptoms of overdosage of glycopyrrolate are peripheral in nature rather than central.
- To guard against further absorption of the drug-use gastric lavage, cathartics and/or enemas.
- To combat peripheral anticholinergic effects (residual mydriasis, dry mouth, etc.)-utilize a quaternary ammonium anticholinesterase, such as neostigmine methylsulfate.
- To combat hypotension-use pressor amines (norepinephrine, metaraminol) i.v.; and supportive care.
- To combat respiratory depression-administer oxygen; utilize a respiratory stimulant such as Dopram®* i.v.; artificial respiration.
-
DOSAGE AND ADMINISTRATION
The dosage of glycopyrrolate should be adjusted to the needs of the individual patient to assure symptomatic control with a minimum of adverse reactions. The presently recommended maximum daily dosage of glycopyrrolate is 8 mg.
Glycopyrrolate Tablets 1 mg. The recommended initial dosage of glycopyrrolate for adults is one tablet three times daily (in the morning, early afternoon, and at bedtime). Some patients may require two tablets at bedtime to assure overnight control of symptoms. For maintenance, a dosage of one tablet twice a day is frequently adequate.
Glycopyrrolate Tablets 2 mg. The recommended dosage of glycopyrrolate for adults is one tablet two or three times daily at equally spaced intervals.
Glycopyrrolate tablets are not recommended for use in pediatric patients under the age of 12 years.
- DRUG INTERACTIONS
-
HOW SUPPLIED
Glycopyrrolate tablets 1 mg are bisected, compressed white, round tablets debossed “K” above the bisect and “400” below the bisect on one side of the tablet, and plain on the other side.
Available in bottles of 100 (NDC 49884-065-01).
Glycopyrrolate tablets 2 mg are bisected, compressed white, round tablets debossed “K” above the bisect and “401” below the bisect on one side of the tablet, and plain on the other side.
Available in bottles of 100 (NDC 49884-066-01).
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
Dispense in tight container.
Rx only
* Dopram® is a registered trademark of Baxter Healthcare Corporation.
Manufactured by:
Par Pharmaceutical Companies, Inc.
Spring Valley, NY 10977
Rev: 07/2010
OS065-01-1-02
- PRINCIPAL DISPLAY PANEL - 1MG/100'S LABEL
- PRINCIPAL DISPLAY PANEL - 2MG/100'S LABEL
-
INGREDIENTS AND APPEARANCE
GLYCOPYRROLATE
glycopyrrolate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49884-065 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCOPYRROLATE (UNII: V92SO9WP2I) (GLYCOPYRRONIUM - UNII:A14FB57V1D) GLYCOPYRROLATE 1 mg Inactive Ingredients Ingredient Name Strength CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code K;400 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49884-065-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040653 09/26/2006 GLYCOPYRROLATE
glycopyrrolate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49884-066 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCOPYRROLATE (UNII: V92SO9WP2I) (GLYCOPYRRONIUM - UNII:A14FB57V1D) GLYCOPYRROLATE 2 mg Inactive Ingredients Ingredient Name Strength CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code K;401 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49884-066-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040653 09/26/2006 Labeler - Par Pharmaceutical, Inc. (092733690)




