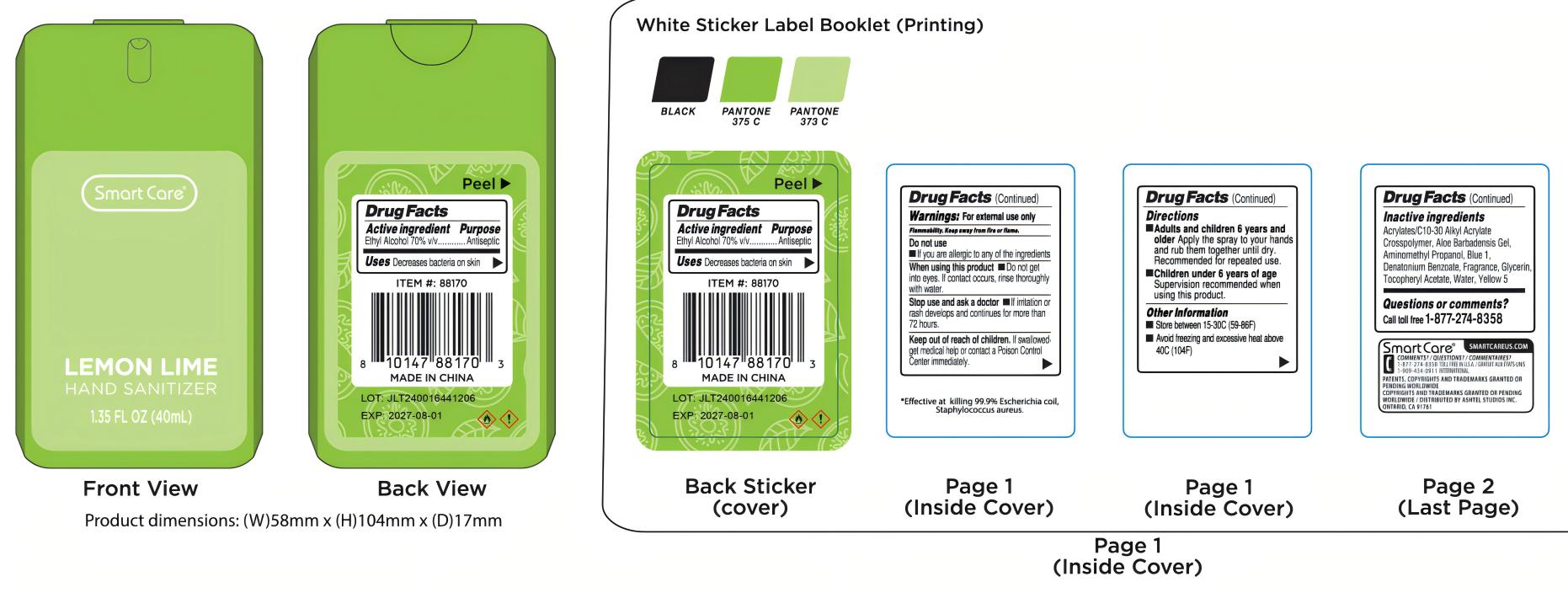
Label: LEMON LIME HAND SANITIZER SPRAY. 01- alcohol spray
- NDC Code(s): 54860-427-01
- Packager: Shenzhen Lantern Scicence Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
- Warning
- When using this product
- Do not use
- Stop use and ask a doctor
- keep out of reach of children
- Directions
- Inactive ingredients
- other Information
- DOSAGE & ADMINISTRATION
- packing
-
INGREDIENTS AND APPEARANCE
LEMON LIME HAND SANITIZER SPRAY. 01
alcohol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54860-427 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength AMINOMETHYLPROPANOL (UNII: LU49E6626Q) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54860-427-01 40 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 08/06/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 08/06/2024 Labeler - Shenzhen Lantern Scicence Co.,Ltd. (421222423) Establishment Name Address ID/FEI Business Operations Shenzhen Lantern Science Co.,Ltd. 421222423 manufacture(54860-427)