Label: NEXGARD PLUS- afoxolaner, moxidectin and pyrantel tablet, chewable
-
NDC Code(s):
0010-4260-01,
0010-4260-02,
0010-4260-03,
0010-4261-01, view more0010-4261-02, 0010-4261-03, 0010-4262-01, 0010-4262-02, 0010-4262-03, 0010-4263-01, 0010-4263-02, 0010-4263-03, 0010-4264-01, 0010-4264-02, 0010-4264-03
- Packager: Boehringer Ingelheim Animal Health USA Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated March 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Caution:
-
Description:
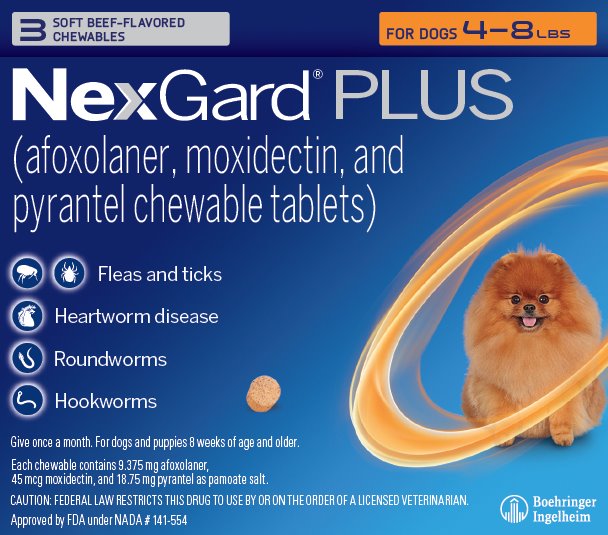
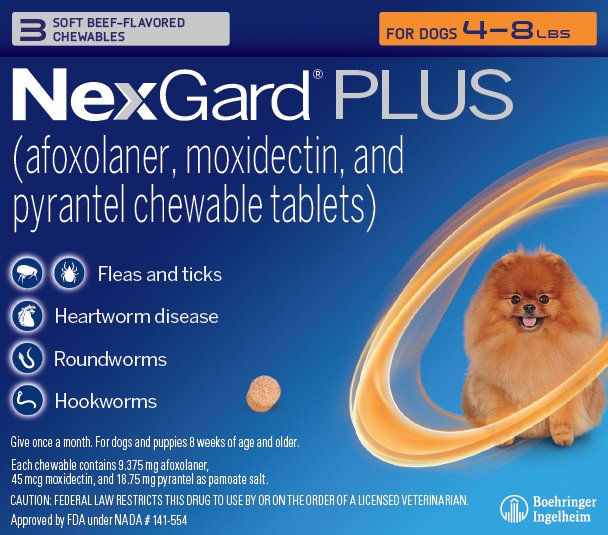
NexGard® PLUS (afoxolaner, moxidectin, and pyrantel chewable tablets) is available in five sizes of beef-flavored, soft chewables for oral administration to dogs and puppies according to their weight. Each chewable is formulated to provide minimum doses of 1.14 mg/lb (2.5 mg/kg) afoxolaner, 5.45 mcg/lb (12 mcg/kg) moxidectin, and 2.27 mg/lb (5.0 mg/kg) pyrantel (as pamoate salt).
Afoxolaner is a member of the isoxazoline family of compounds. Its chemical name is 1-Naphthalenecarboxamide,4-[5-[3-chloro-5-(trifluoromethyl)-phenyl]-4,5-dihydro-5-(trifluoromethyl)-3-isoxazolyl]-N-[2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl.
Moxidectin is a semisynthetic macrocyclic lactone derived from the actinomycete Streptomycetes cyaneogriseus noncyanogenus. The chemical name for moxidectin is [6R,23E,25S(E)]-5-O-Demethyl-28-deoxy-25-(1,3-dimethyl-1-butenyl)-6,28-epoxy-23-(methoxyimino) milbemycin B.
Pyrantel is a member of the tetrahydropyrimidine family of compounds. Its chemical name is (E)-1,4,5,6-Tetrahydro-1-methyl-2-[2-(2-thienyl) vinyl] pyrimidine 4,4’methylenebis [3-hydroxy-2-naphthoate](1:1).
-
Indications:
NexGard® PLUS is indicated for the prevention of heartworm disease caused by Dirofilaria immitis and for the treatment and control of adult hookworm (Ancylostoma caninum, Ancylostoma braziliense, and Uncinaria stenocephala) and roundworm (Toxocara canis and Toxascarisleonina) infections. NexGard® PLUS kills adult fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) and the treatment and control of Ixodesscapularis (black-legged tick), Rhipicephalus sanguineus (brown dog tick), Dermacentor variabilis (American dog tick), and Amblyomma americanum (lone star tick) infestations for one month in dogs and puppies eight weeks of age and older, weighing four pounds of body weight or greater.
-
Dosage and Administration:
NexGard® PLUS is given orally once a month at the minimum dosage of 1.14 mg/lb (2.5 mg/kg) afoxolaner, 5.45 mcg/lb (12 mcg/kg) moxidectin, and 2.27 mg/lb (5.0 mg/kg) pyrantel (as pamoate salt).
For heartworm disease prevention, give once monthly for at least six months after last exposure to mosquitoes (see Effectiveness).
-
Dosing Schedule:
Body
Weight
(lbs.)
Afoxolaner
Per
Chewable
(mg)
Moxidectin
Per
Chewable
(µg)
Pyrantel*
Per
Chewable
(mg)
Chewables
Administered
4 - 8
9.375
45
18.75
One
8.1 - 17
18.75
90
37.5
One
17.1 - 33
37.5
180
75
One
33.1 - 66
75
360
150
One
66.1 - 132
150
720
300
One
Over 132
Administer the appropriate combination of chewables
*As pamoate salt.
NexGard® PLUS can be administered with or without food. Care should be taken to ensure that the dog consumes the complete dose and that part of the dose is not lost or refused. If a dose is missed, administer NexGard® PLUS and resume a monthly dosing schedule.
Heartworm Prevention:
NexGard® PLUS should be administered at monthly intervals year-round or, at a minimum, administration should start within one month of the dog’s first seasonal exposure to mosquitoes and should continue at monthly intervals until at least six months after the dog’s last exposure (see Effectiveness). When replacing another monthly heartworm preventive product, the first dose of NexGard® PLUS should be given within a month of the last dose of the former medication.
Flea Treatment and Prevention:
NexGard® PLUS should be administered year-round at monthly intervals or started at least one month before fleas become active. To minimize the likelihood of flea reinfestation, it is important to treat all animals within a household with an approved flea control product.
Tick Treatment and Control:
NexGard® PLUS should be administered year-round at monthly intervals or started at least one month before ticks become active.
Intestinal Nematode Treatment and Control:
NexGard® PLUS treats and controls adult hookworms (Ancylostoma caninum, Ancylostoma braziliense, and Uncinaria stenocephala) and roundworms (Toxocara canis and Toxascaris leonina). For the treatment of adult hookworm and roundworm infections, NexGard® PLUS should be administered as a single dose. Monthly use of NexGard® PLUS will control any subsequent infections. Dogs may be exposed to and can become infected with hookworms and roundworms throughout the year, regardless of season or climate.
- Contraindications:
- Warnings:
-
Precautions:
Afoxolaner, one of the ingredients in NexGard® PLUS, is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and seizures. Seizures have been reported in dogs receiving isoxazoline class drugs, even in dogs without a history of seizures. Use with caution in dogs with a history of seizures or neurologic disorders.
Treatment with fewer than six monthly doses after the last exposure to mosquitoes has not been evaluated and may not provide complete heartworm prevention.
Prior to administration of NexGard® PLUS, dogs should be tested for existing heartworm infection. At the discretion of the veterinarian, infected dogs should be treated with an adulticide to remove adult heartworms. NexGard® PLUS is not effective against adult D. immitis.
The safe use of NexGard® PLUS in breeding, pregnant, or lactating dogs has not been evaluated.
-
Adverse Reactions:
In a field safety and effectiveness study, NexGard® PLUS was administered to dogs for the prevention of heartworm disease. The study included a total of 272 dogs (134 administered NexGard® PLUS and 138 administered active control) treated once monthly for 11 treatments. Over the 330-day study period, all observations of potential adverse reactions were recorded. The most frequent reactions reported in the NexGard® PLUS group are presented in the following table.
Table 1. Dogs With Adverse Reactions
Clinical Sign
NexGard® PLUS
n = 134
Number (Percentage)
Active Control
n = 138
Number (Percentage)
Diarrhea
9 (6.7%)
7 (5.1%)
Vomiting
6 (4.5%)
7 (5.1%)
Lethargy
3 (2.2%)
5 (3.6%)
Itching
3 (2.2%)
3 (2.2%)
Dermatitis
2 (1.5%)
1 (0.7%)
Anorexia
1 (0.7%)
4 (2.9%)
Muscle tremor
1 (0.7%)
1 (0.7%)
One dog in the NexGard® PLUS group was reported to exhibit muscle tremors along with nausea and depression for one day after the Day 0 treatment. The dog remained in the study and muscle tremors were not reported after any subsequent treatments.
-
Contact Information:
For a copy of the Safety Data Sheet (SDS) or to report suspected adverse drug events, contact Boehringer Ingelheim Animal Health USA Inc. at 1-888-637-4251 or www.nexgardforpets.com.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
-
Clinical Pharmacology:
Mode of Action:
NexGard® PLUS (afoxolaner, moxidectin, and pyrantel chewable tablets) contains the three active pharmaceutical ingredients afoxolaner, moxidectin, and pyrantel (as pamoate salt).
Afoxolaner is a member of the isoxazoline family, shown to bind at a binding site to inhibit insect and acarine ligand-gated chloride channels, in particular those gated by the neurotransmitter gamma-aminobutyric acid (GABA), thereby blocking pre- and postsynaptic transfer of chloride ions across cell membranes. Prolonged afoxolaner-induced hyperexcitation results in uncontrolled activity of the central nervous system and death of insects and acarines. The selective toxicity of afoxolaner between insects and acarines and mammals may be inferred by the differential sensitivity of the insects and acarines’ GABA receptors versus mammalian GABA receptors.
Moxidectin is an endectocide in the macrocyclic lactone class. Moxidectin acts by interfering with chloride channel-mediated neurotransmission in susceptible parasites, which results in paralysis and death of the parasite.
Pyrantel is a nematocide belonging to the tetrahydropyrimidine class. Pyrantel acts as a depolarizing, neuromuscular-blocking agent in susceptible parasites, causing paralysis and death or expulsion of the parasite.
Pharmacokinetics:
Following a single oral administration of a near-final formulation of NexGard® PLUS (at mean doses of 3.9 mg/kg afoxolaner, 18.8 mcg/kg moxidectin, and 7.8 mg/kg pyrantel pamoate) in fed and fasted Beagle dogs (10 to 21 months of age), afoxolaner and moxidectin were more rapidly absorbed in the fasted state with a time to maximum concentration (Tmax) of 2 to 3 hours. The afoxolaner mean maximum plasma concentrations (Cmax) in the fed and fasted states were 1610 and 2200 ng/mL (CV=33 and 16%) and the moxidectin mean Cmax values were 11.1 and 15.5 ng/mL (CV=39 and 24%), respectively. The area under the curve (AUC) for afoxolaner and moxidectin were similar between fed and fasted states. Post-dose pyrantel plasma concentrations were quantifiable out to 24 hours.
Following six oral administrations of NexGard® PLUS at 1, 3 and 5X the maximum exposure dose of 5 mg/kg, 24 mcg/kg, and 10 mg/kg afoxolaner, moxidectin and pyrantel pamoate, respectively, every 28 days in 8-week-old Beagle dogs, afoxolaner and moxidectin Tmax ranged from 2 to 6 hours. The observed mean Cmax and AUC at steady state in the 1X dose group were 2230 ng/mL and 19000 days*ng/mL for afoxolaner and 14.8 ng/mL and 55.2 days*ng/mL for moxidectin, respectively. Based on mean Cmin, afoxolaner and moxidectin accumulated by less than 4-fold at steady state. Afoxolaner and moxidectin exposure increased in a dose proportional manner between the 1X and 3X dose groups but was less than dose proportional in the 5X dose group.
Pyrantel pamoate is poorly absorbed into systemic circulation. Pyrantel pamoate is intended to remain in the gastrointestinal tract to allow effective concentrations to be delivered to gastrointestinal nematodes.
-
Effectiveness:
Heartworm Prevention:
In two well-controlled laboratory studies, NexGard® PLUS was 100% effective against induced D. immitis infections when administered for six consecutive months.
In a well-controlled US field study consisting of 120 dogs administered NexGard® PLUS and 124 administered an active control, no dogs treated with NexGard® PLUS tested positive for heartworm disease. All dogs treated with NexGard® PLUS were negative for D. immitis antigen and blood microfilariae at study completion on Day 330.
Flea Treatment and Prevention:
In a well-controlled laboratory study, NexGard® PLUS demonstrated ≥99.8% effectiveness against adult fleas 24 hours after weekly infestations for one month.
In a separate well-controlled laboratory study, afoxolaner alone began to kill fleas four hours after initial administration and demonstrated >99% effectiveness at eight hours.
In an additional well-controlled laboratory study, afoxolaner alone demonstrated 100% effectiveness against adult fleas 24 hours post-infestation for 35 days and was ≥93% effective at 12 hours post-infestation through Day 21 and on Day 35. On Day 28, afoxolaner alone was 81.1% effective 12 hours post-infestation. Dogs in both the afoxolaner-treated and control groups that were infested with fleas on Day -1 generated flea eggs at 12 and 24 hours post-treatment (0-11 eggs and 1-17 eggs in the afoxolaner-treated dogs, and 4-90 eggs and 0-118 eggs in the control dogs, at 12 and 24 hours, respectively). At subsequent evaluations post-infestation, fleas from dogs in the afoxolaner-treated group were essentially unable to produce any eggs (0-1 eggs), while fleas from dogs in the control group continued to produce eggs (1-141 eggs).
In a 90-day US field study conducted in households with existing flea infestations of varying severity, the effectiveness of afoxolaner alone against fleas on the Day 30, 60, and 90 visits compared with baseline was 98.0%, 99.7%, and 99.9%, respectively.
Collectively, the data from the four studies (three laboratory and one field) demonstrate that NexGard® PLUS kills fleas before they can lay eggs, thus preventing subsequent flea infestations after the start of treatment of existing flea infestations.
Tick Treatment and Control:
In well-controlled laboratory studies, afoxolaner alone demonstrated >97% effectiveness against Dermacentor variabilis, >94% effectiveness against Ixodes scapularis, and >93% effectiveness against Rhipicephalus sanguineus, 48 hours post-infestation, for one month. At 72 hours post-infestation, NexGard® PLUS demonstrated ≥97% effectiveness against Amblyomma americanum for one month.
Intestinal Nematode Treatment and Control:
Elimination of adult roundworms (Toxocara canis and Toxascaris leonina) and hookworms (Ancylostoma caninum, Ancylostoma braziliense, and Uncinaria stenocephala) was demonstrated in well-controlled laboratory studies.
-
Target Animal Safety:
Margin of Safety:
NexGard® PLUS was administered orally at 1, 3, and 5X the maximum exposure doses at approximately 28-day intervals for six treatments to 8-week-old Beagle puppies. Dogs in the control group were sham-dosed. There were no clinically relevant, treatment-related effects on body weights, food consumption, clinical pathology (hematology, coagulation, serum chemistry, and urinalysis), gross pathology, histopathology, organ weights, or ophthalmic examinations. Mild, self-limiting diarrhea (with and without blood) was possibly related to treatment, as there were more incidences in the NexGard® PLUS groups than the control group throughout the study, including within 48 hours after treatment.
Avermectin-Sensitive Collie Safety:
NexGard® PLUS was administered orally at 1, 3, and 5X the maximum label dose to MDR1-deficient Collies once on Day 0, with a second administration to the 1X group on Day 28. Dogs in the control group were sham-dosed on Days 0 and 28. No clinical signs of avermectin toxicity were noted in any dog at any time during the study. Vomiting was observed in some dogs in the 3X and 5X groups and resolved without treatment. Diarrhea, with or without blood, was observed in some dogs in all of the NexGard® PLUS groups and resolved without treatment.
Heartworm-Positive Safety:
NexGard® PLUS was administered orally at 1X and 3X the maximum exposure doses at approximately 28-day intervals for three treatments to Beagle dogs with adult heartworm infections and circulating microfilariae. Dogs in the control group were sham-dosed. Diarrhea was observed in one dog in the 1X group and in three dogs in the 3X group, and vomiting was observed in two dogs in the 3X group. No signs of avermectin toxicity were observed at any time during the study. There were no clinical signs associated with death of the microfilariae observed in any of the dogs.
Field Safety:
In a well-controlled field study, NexGard® PLUS was used concurrently with other medications such as vaccines, antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs), anesthetics, sedatives, analgesics, steroids, anthelmintics, antiemetics, and antipruritics. No adverse reactions were associated with the concurrent use of NexGard® PLUS and other medications.
- How Supplied:
- Storage Information:
-
SPL UNCLASSIFIED SECTION
Approved by FDA under NADA # 141-554
Marketed by: Boehringer Ingelheim Animal Health USA Inc., Duluth, GA 30096
NEXGARD® is a registered trademark of Boehringer Ingelheim Animal Health France, used under license.
© 2023 Boehringer Ingelheim Animal Health USA Inc. All rights reserved.
181531-002
Rev. 07/2023
- Principal Display Panel – Display Carton – For Dogs 4 – 8 lbs – 3 chewables
- Principal Display Panel - Display Carton – For Dogs 8.1 - 17 lbs - 3 chewables
- Principal Display Panel - Display Carton – For Dogs 17.1 - 33 lbs - 3 chewables
- Principal Display Panel - Display Carton – For Dogs 33.1 – 66 lbs - 3 chewables
- Principal Display Panel – Display Carton – For Dogs 66.1 – 132 lbs – 3 chewables
-
INGREDIENTS AND APPEARANCE
NEXGARD PLUS
afoxolaner, moxidectin and pyrantel tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0010-4260 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AFOXOLANER (UNII: 02L07H6D0U) (AFOXOLANER - UNII:02L07H6D0U) AFOXOLANER 9.375 mg MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 0.045 mg PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 18.75 mg Product Characteristics Color BROWN (mottled yellowish-brown to reddish-brown with possible marbling) Score no score Shape ROUND Size 10mm Flavor MEAT (Beef) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0010-4260-01 1 in 1 CARTON 1 1 in 1 BLISTER PACK 2 NDC:0010-4260-02 1 in 1 CARTON 2 3 in 1 BLISTER PACK 3 NDC:0010-4260-03 1 in 1 CARTON 3 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141554 11/13/2023 NEXGARD PLUS
afoxolaner, moxidectin and pyrantel tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0010-4261 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AFOXOLANER (UNII: 02L07H6D0U) (AFOXOLANER - UNII:02L07H6D0U) AFOXOLANER 18.75 mg MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 0.090 mg PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 37.5 mg Product Characteristics Color BROWN (mottled yellowish-brown to reddish-brown with possible marbling) Score no score Shape RECTANGLE Size 13mm Flavor MEAT (Beef) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0010-4261-01 1 in 1 CARTON 1 1 in 1 BLISTER PACK 2 NDC:0010-4261-02 1 in 1 CARTON 2 3 in 1 BLISTER PACK 3 NDC:0010-4261-03 1 in 1 CARTON 3 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141554 07/20/2023 NEXGARD PLUS
afoxolaner, moxidectin and pyrantel tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0010-4262 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AFOXOLANER (UNII: 02L07H6D0U) (AFOXOLANER - UNII:02L07H6D0U) AFOXOLANER 37.5 mg MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 0.18 mg PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 75 mg Product Characteristics Color BROWN (mottled yellowish-brown to reddish-brown with possible marbling) Score no score Shape RECTANGLE Size 15mm Flavor MEAT (Beef) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0010-4262-01 1 in 1 CARTON 1 1 in 1 BLISTER PACK 2 NDC:0010-4262-02 1 in 1 CARTON 2 3 in 1 BLISTER PACK 3 NDC:0010-4262-03 1 in 1 CARTON 3 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141554 07/20/2023 NEXGARD PLUS
afoxolaner, moxidectin and pyrantel tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0010-4263 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AFOXOLANER (UNII: 02L07H6D0U) (AFOXOLANER - UNII:02L07H6D0U) AFOXOLANER 75 mg MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 0.36 mg PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 150 mg Product Characteristics Color BROWN (mottled yellowish-brown to reddish-brown with possible marbling) Score no score Shape RECTANGLE Size 22mm Flavor MEAT (Beef) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0010-4263-01 1 in 1 CARTON 1 1 in 1 BLISTER PACK 2 NDC:0010-4263-02 1 in 1 CARTON 2 3 in 1 BLISTER PACK 3 NDC:0010-4263-03 1 in 1 CARTON 3 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141554 07/20/2023 NEXGARD PLUS
afoxolaner, moxidectin and pyrantel tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0010-4264 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AFOXOLANER (UNII: 02L07H6D0U) (AFOXOLANER - UNII:02L07H6D0U) AFOXOLANER 150 mg MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 0.72 mg PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 300 mg Product Characteristics Color BROWN (mottled yellowish-brown to reddish-brown with possible marbling) Score no score Shape RECTANGLE Size 32mm Flavor MEAT (Beef) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0010-4264-01 1 in 1 CARTON 1 1 in 1 BLISTER PACK 2 NDC:0010-4264-02 1 in 1 CARTON 2 3 in 1 BLISTER PACK 3 NDC:0010-4264-03 1 in 1 CARTON 3 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141554 07/20/2023 Labeler - Boehringer Ingelheim Animal Health USA Inc. (007134091)