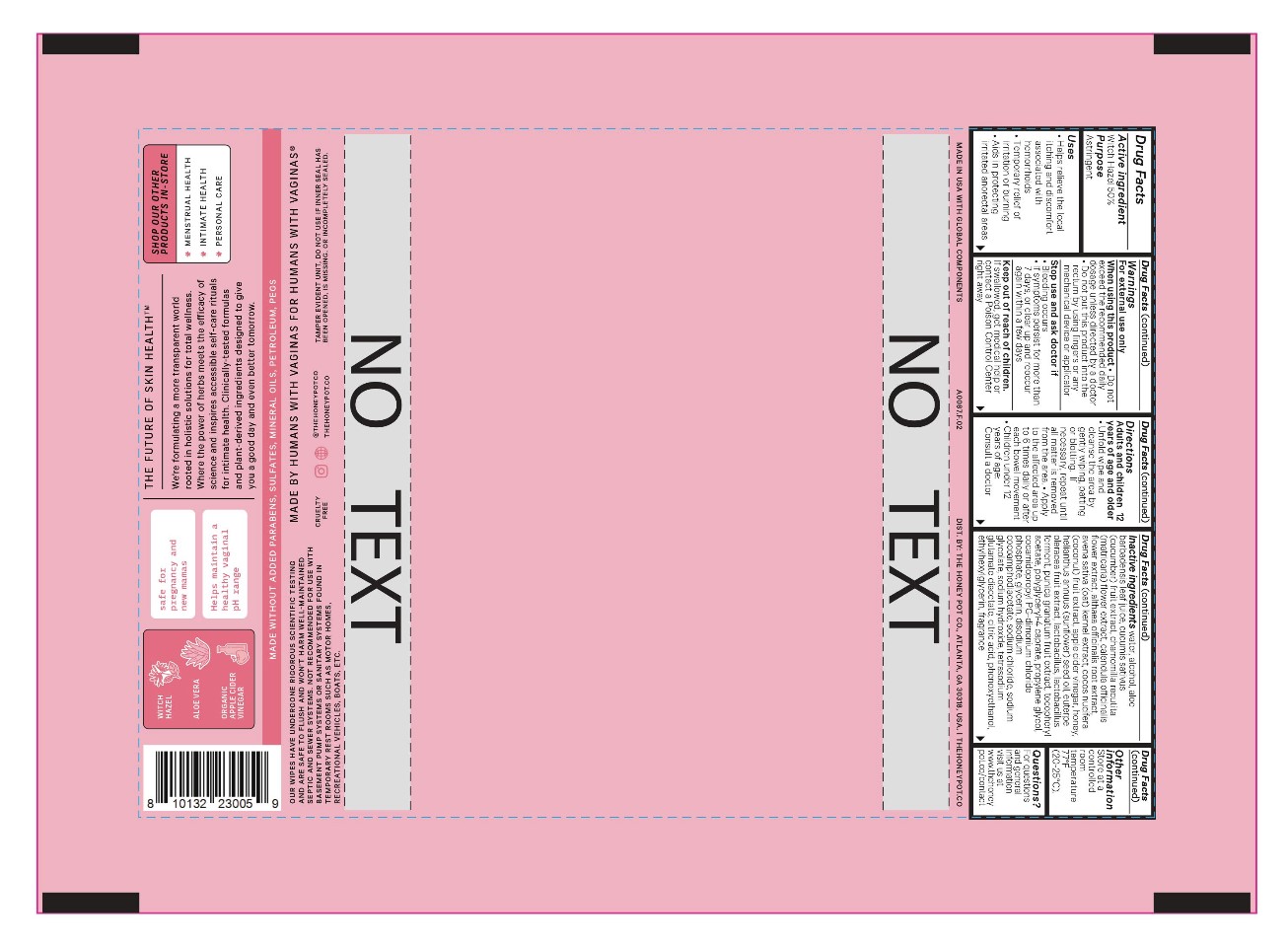
Label: SOOTHING HEMORRHOIDAL WIPES 50 WITCH HAZEL- witch hazel cloth
- NDC Code(s): 82637-0059-1
- Packager: The Honey Pot Company LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient:
- Uses:
-
Warnings:
For external use only
When using this product:
- Do not exceed the recommended daily dosage unless directed by a doctor
- Do not put this product into the rectum by using fingers or any mechanical device or applicator
-
Directions:
Adults and children 12 years of age and older:
- Unfold wipe and cleanse the area by gently wiping, patting or blotting. If necessary, repeat until all matter is removed from the area
- Apply to the affected area up to 6 times daily or after each bowel movement
Children under 12 years of age: Consult a doctor
-
Inactive Ingredients:
water, alcohol, aloe barbadensis leaf juice, cucumis sativus (cucumber) fruit extract, chamomilla recutita (matricaria) flower extract, calendula officinalis flower extract, althaea officinalis root extract, avena sativa (oat) kernel extract, cocos nucifera (coconut) fruit extract, apple cider vinegar, honey, helianthus annuus (sunflower) seed oil, euterpe oleracea fruit extract, lactobacillus, lactobacillus ferment, punica granatum fruit extract, tocopheryl acetate, polyglyceryl-4 caprate, propylene glycol, cocamidopropyl PG-dimonium chloride phosphate, glycerin, disodium cocoamphodiacetate, sodium chloride, sodium glycolate, sodium hydroxide, tetrasodium glutamate diacetate, citric acid, phenoxyethanol, ethylhexylglycerin, fragrance
- Other Information:
- Questions:
- Prinicpal Display Panel:
-
INGREDIENTS AND APPEARANCE
SOOTHING HEMORRHOIDAL WIPES 50 WITCH HAZEL
witch hazel clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82637-0059 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WITCH HAZEL (UNII: 101I4J0U34) (WITCH HAZEL - UNII:101I4J0U34) WITCH HAZEL 500 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) LIMOSILACTOBACILLUS FERMENTUM (UNII: 2C1F12C6AP) ALOE VERA LEAF (UNII: ZY81Z83H0X) OAT (UNII: Z6J799EAJK) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CHAMOMILE (UNII: FGL3685T2X) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) COCONUT (UNII: 3RT3536DHY) CUCUMBER (UNII: YY7C30VXJT) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ACAI (UNII: 46AM2VJ0AW) GLYCERIN (UNII: PDC6A3C0OX) SUNFLOWER OIL (UNII: 3W1JG795YI) HONEY (UNII: Y9H1V576FH) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 CAPRATE (UNII: 3N873UN885) POMEGRANATE (UNII: 56687D1Z4D) SODIUM CHLORIDE (UNII: 451W47IQ8X) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) APPLE CIDER VINEGAR (UNII: 0UE22Q87VC) ALTHAEA OFFICINALIS ROOT (UNII: TRW2FUF47H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BIFIDOBACTERIUM ANIMALIS LACTIS (UNII: 5307V7XW8I) ALCOHOL (UNII: 3K9958V90M) SODIUM GLYCOLATE (UNII: B75E535IMI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82637-0059-1 30 in 1 POUCH 09/01/2024 1 2.8 mL in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 09/01/2024 Labeler - The Honey Pot Company LLC (045600502)