Label: APLISOL- tuberculin purified protein derivative injection
- NDC Code(s): 42023-104-01, 42023-104-05
- Packager: Par Pharmaceutical, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 31, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Aplisol (tuberculin PPD, diluted) is a sterile aqueous solution of a purified protein fraction for intradermal administration as an aid in the diagnosis of tuberculosis. The solution is stabilized with polysorbate (Tween) 80, buffered with potassium and sodium phosphates and contains approximately 0.25% phenol as a preservative.
This product is ready for immediate use without further dilution.
The purified protein fraction is isolated from culture media filtrates of a human strain of Mycobacterium tuberculosis by the method of F.B. Seibert.1,2 Tuberculin PPD, diluted, is prepared from Tuberculin PPD which is clinically bioequivalent in potency to the standard PPD-S1(5 TU2per 0.1mL) of the U.S. Public Health Service, National Centers for Disease Control.
The potency of each lot of tuberculin PPD, diluted is determined in sensitized guinea pigs.
-
CLINICAL PHARMACOLOGY
In the United States, the prevalence of Mycobacterium tuberculosis infection and active disease varies for different segments of the population; however, the risk for M. tuberculosis infection in the overall population is low. Tuberculosis (TB) case rates declined steadily for decades in the United States. However, in 1985 the TB case rate stabilized and subsequently increased through 1992, accompanied by a 14% increase in the TB mortality rate in 1988. This has been attributed to several complex social and medical factors, including the human immunodeficiency virus (HIV) epidemic, the occurrence of TB in foreign-born persons from countries that have a high prevalence of TB, the emergence of drug-resistant strains of TB, and the transmission of M. tuberculosis in congregate settings (e.g., health-care facilities, correctional facilities, drug-treatment centers, and homeless shelters). Because the overall risk of acquiring M. tuberculosis is low for the total U.S. population, the primary strategy for preventing and controlling TB in the United States is to minimize the risk of transmission by the early identification and treatment of patients who have active infectious TB, finding and screening persons who have been in contact with active infectious TB patients and screening high risk populations.
Tuberculin PPD is indicated as an aid in the detection of infection with Mycobacterium tuberculosis.3,4 After a person becomes infected with mycobacteria, T lymphocytes proliferate and become sensitized. These sensitized T cells enter the bloodstream and circulate for months or years. This sensitization process occurs principally in the regional lymph nodes and may take 2–10 weeks to develop following infection. Once acquired, tuberculin sensitivity tends to persist, although it often wanes with time and advancing age. The injection of tuberculin into the skin stimulates the lymphocytes and activates the series of events leading to a delayed-type hypersensitivity (DTH) response. This response is called "delayed" because the reaction becomes evident hours after injection. Dermal reactivity involves vasodilation, edema, and the infiltration of lymphocytes, basophils, monocytes, and neutrophils into the site of antigen injection. Antigen-specific T lymphocytes proliferate and release lymphokines, which mediate the accumulation of other cells at the site. The area of induration reflects DTH activity.5 In most tuberculin-sensitive individuals, the delayed hypersensitivity reaction is evident 5–6 hours after administration of a tuberculin skin test and is maximal 48–72 hours. In geriatric patients or in patients receiving a tuberculin skin test for the first time, the reaction may develop more slowly and may not be maximal until after 72 hours.6,7 Because their immune systems are immature, many neonates and infants <6 weeks of age, who are infected with M. tuberculosis, do not react at all to tuberculin tests.5
Immediate erythematous or other hypersensitivity reactions to tuberculin or the constituents of the diluent may occur at the injection site.
A possible decrease in responsiveness to skin testing may occur in the presence of infections, viral infections (measles, mumps, chickenpox, HIV), live virus vaccinations (measles, mumps, rubella, oral polio, varicella, yellow fever), bacterial infections (typhoid fever, brucellosis, typhus, leprosy, pertussis, overwhelming tuberculosis, tuberculous pleurisy), fungal infections (South American blastomycosis), drugs (corticosteroids and other immunosuppressive agents), metabolic derangements (chronic renal failure), low protein states (severe protein depletion, afibrinogenemia), age (newborns, elderly patients with waned sensitivity), stress (surgery, burns, mental illness, graft-versus-host reactions), diseases affecting lymphoid organs (Hodgkin's disease, lymphoma, chronic leukemia, sarcoidosis) and malignancy (see WARNINGS).
Tuberculin skin-test results are also less reliable as CD4 counts decline in HIV infected individuals.3
The 5TU dose of Tuberculin PPD intradermally (Mantoux) is indicated as an aid in the detection of infection with Mycobacterium tuberculosis. Reactions to the Mantoux test are interpreted on the basis of a quantitative measurement of the response to a specific dose (5 TU PPD-S or equivalent) of Tuberculin PPD.7
To determine that the Tuberculin PPD is clinically bioequivalent in potency to standard 5TU PPD-S1, 3 dose-response studies were conducted in the following populations (1) persons with a history of bacteriologically confirmed TB; (2) healthy volunteers; and (3) volunteers with active or previously active nontuberculosis mycobacterial lung disease.
-
INDICATIONS AND USAGE
Tuberculin PPD is indicated as an aid in the detection of infection with Mycobacterium tuberculosis. The standard tuberculin test employs the intradermal (Mantoux) test using a 5 TU dose of tuberculin PPD.7 The 0.1 mL test dose of Aplisol (tuberculin PPD, diluted) is equivalent to the 5 TU dose which has been clinically utilized and standardized with PPD-S. Tuberculin skin testing is not contraindicated for persons who have been vaccinated with BCG and the skin-test results of such persons are used to support or exclude the diagnosis of M. tuberculosis infections.4 HIV infection is a strong risk factor for the development of TB disease in persons having TB infection. All HIV-infected persons should receive a PPD-tuberculin skin test.3
-
CONTRAINDICATIONS
Aplisol is contraindicated in patients with known hypersensitivity or allergy to Aplisol or any of its components. Aplisol should not be administered to persons who have previously experienced a severe reaction (e.g., vesiculation, ulceration, or necrosis) because of the severity of reactions that may occur at the test site.
-
WARNINGS
Aplisol should not be administered to persons who previously experienced a severe reaction (e.g., vesiculation, ulceration, or necrosis) because of the severity of reactions that may occur at the test site (see CONTRAINDICATIONS).
Not all infected persons will have a delayed hypersensitivity reaction to a tuberculin test. A number of factors have been reported to cause a decreased ability to respond to the tuberculin test, such as the presence of infections, viral infections (measles, mumps, chickenpox, HIV), live virus vaccinations (measles, mumps, rubella and other live vaccines), bacterial infections (typhoid fever, brucellosis, typhus, leprosy, pertussis, overwhelming tuberculosis, tuberculous pleurisy), fungal infections (South American blastomycosis), drugs (corticosteroids and other immunosuppressive agents), metabolic derangements (chronic renal failure), low protein states (severe protein depletion, afibrinogenemia), age (newborns, elderly patients with waned sensitivity), stress (surgery, burns, mental illness, graft-versus-host reactions), diseases affecting lymphoid organs (Hodgkin's disease, lymphoma, chronic leukemia, sarcoidosis), and malignancy.7,8,9
Any condition that impairs or attenuates cell mediated immunity potentially can cause a false negative reaction, including aging.10,11
Tuberculin skin test results are less reliable in HIV-infected individuals as CD4 counts decline (see CLINICAL PHARMACOLOGY).3
Avoid injecting tuberculin subcutaneously. If this occurs, no local reaction develops, but a general febrile reaction and/or acute inflammation around old tuberculous lesions may occur in highly sensitive individuals.
-
PRECAUTIONS
General
The predictive value of the tuberculin skin test depends on the prevalence of infection with M. tuberculosis and the relative prevalence of cross-reactions with nontuberculous mycobacteria.9,12
A separate, sterile, single-use disposable syringe and needle should be used for each individual patient to prevent possible transmission of serum hepatitis virus and other infectious agents from one person to another. Special care should be taken to ensure that the product is injected intradermally and not into a blood vessel.
Before administration of Aplisol, a review of the patient's history with respect to possible immediate-type hypersensitivity to the product, determination of previous use of Aplisol and the presence of any contraindication to the test should be made (see CONTRAINDICATIONS).
As with any biological product, epinephrine should be immediately available in case an anaphylactoid or acute hypersensitivity reaction occurs.
Failure to store and handle Aplisol as recommended may result in a loss of potency and inaccurate test results.8,13
Reactivity to the test may be depressed or suppressed for as long as 5–6 weeks in individuals following immunization with certain live viral vaccines, viral infections or discontinuation of corticosteroids or immunosuppressive agents.8,9
Information to Patients
Patients should be instructed to report adverse events such as vesiculation, ulceration or necrosis which may occur at the test site in highly sensitive individuals. Patients should be informed that pain, pruritus and discomfort may occur at injection site.
Patients should be informed of the need to return to their physician or health care provider for the reading of the test and of the need to keep and maintain a personal immunization record.
Drug Interactions
In patients who are receiving corticosteroids or immunosuppressive agents, reactivity to the test may be depressed or suppressed. This reduced reactivity may be present for as long as 5–6 weeks after discontinuation of therapy (see PRECAUTIONS – General).9
The reactivity to PPD may be temporarily depressed by certain live virus vaccines (measles, mumps, rubella, oral polio, yellow fever, and varicella). Therefore, if a tuberculin test is to be performed, it should be administered either before the live vaccine or given simultaneously, but at a separate site than the live vaccine, or testing should be postponed for 4–6 weeks.9
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long term studies have been conducted in animals or in humans to evaluate carcinogenic or mutagenic potential or effects on fertility with Aplisol.
Pregnancy
Teratogenic effects
Pregnancy Category C
Animal reproduction studies have not been conducted with Aplisol. It is also not known whether Aplisol can cause fetal harm when administered to a pregnant woman or can affect the reproduction capacity. Aplisol should be given to a pregnant woman only if clearly needed. However, the risk of unrecognized tuberculosis and the postpartum contact between a mother with active disease and an infant leaves the infant in grave danger of tuberculosis and complications such as tuberculous meningitis. Although there have not been any reported adverse effects upon the fetus recognized as being due to tuberculosis skin testing, the prescribing physician will want to consider if the potential benefits outweigh the possible risks for performing the tuberculin test on a pregnant woman or a woman of childbearing age, particularly in certain high risk populations.
Tuberculin skin testing is considered valid and safe throughout pregnancy.3
Geriatric Use
Once acquired, tuberculin sensitivity tends to persist, although it often wanes with time and advancing age. In geriatric patients or in patients receiving a tuberculin skin test for the first time, the reaction may develop more slowly and may not be maximal until after 72 hours.6,7 (see CLINICAL PHARMACOLOGY). Not all infected persons will have a delayed hypersensitivity reaction to a tuberculin test. A number of factors have been reported to cause a decreased ability to respond to the tuberculin test, such as elderly patients with waned sensitivity.7 Any condition that impairs or attenuates cell mediated immunity potentially can cause a false negative reaction, including aging10,11 (see WARNINGS). An induration of >10 mm is classified as positive in all persons who do not meet any of the criteria listed under an induration of >5 mm, but who belong to one or more of the following groups at high risk for TB, including residents and employees of high risk congregate settings, such as nursing homes and other long-term facilities for the elderly.
The negative tuberculin skin test should never be used to exclude the possibility of active tuberculosis among person for whom the diagnosis is being considered (symptoms compatible with tuberculosis) (see DOSAGE AND ADMINISTRATION-Interpretation of Tuberculin Reaction).
Pediatric Use
Because their immune systems are immature, many neonates and infants <6 weeks of age, who are infected with M. tuberculosis, may not have a delayed hypersensitivity reaction to a tuberculin test (see WARNINGS). Older infants and children develop tuberculin sensitivity 3-6 weeks, and up to 3 months, after initial infection.5,20 Infants and children who have been exposed to persons with active tuberculosis should be considered positive when reaction to the tuberculin skin test measures ≥ 5 mm. Those children younger than 4 years of age who are exposed to persons at increased risk to acquire tuberculosis are considered positive when reaction measures ≥10 mm. Children with minimal risk exposure to tuberculosis would be considered positive when reaction measures ≥15 mm. 5,20 Other criteria for positive tuberculin reactions that are applicable to both pediatric and adult patients are provided in DOSAGE AND ADMINISTRATION, Interpretation of Tuberculin Reaction.
-
ADVERSE REACTIONS
In highly sensitive individuals, strongly positive reactions including vesiculation, ulceration or necrosis may occur at the test site. Cold packs or topical steroid preparations may be employed for symptomatic relief of the associated pain, pruritus and discomfort.
Strongly positive test reactions may result in scarring at the test site.
Immediate erythematous or other reactions may occur at the injection site.
Local hypersensitivity reactions may occur at the injection site including erythema, pruritus, edema, urticaria and rash.
Systemic allergic reactions including anaphylaxis/anaphylactoid reactions have been reported to occur in association with the use of Aplisol. The reactions, including anaphylaxis, generally occurred within 24 hours of exposure and manifestations included rash, urticaria, edema/angioedema and pruritus.
To report SUSPECTED ADVERSE REACTIONS, contact Par Pharmaceutical at 1-800-828-9393 or FDA at 1-888-INFO-FDA (1-888-463-6332) or www.fda.gov/medwatch/.
-
DOSAGE AND ADMINISTRATION
Aplisol vials should be inspected visually for both particulate matter and discoloration prior to administration and discarded if either is seen. Vials in use for more than 30 days should be discarded.
The 0.1 mL dose of Aplisol (tuberculin PPD, diluted) is equivalent to the 5 tuberculin units (TU) dose of Tuberculin PPD, which is the standard strength used for intradermal Mantoux testing.
Standard Method (Mantoux Test)
The Mantoux test is performed by intradermally injecting, on the volar aspect of the forearm, with a syringe and needle, exactly 0.1 mL of Aplisol. The result is read 48 to 72 hours later and palpable induration only is considered in interpreting the test. Induration is a hard, raised area with clearly defined margins at and around the injection site (see Interpretation of Tuberculin Reaction). Erythema may develop at the injection site but has no diagnostic value.
The standard test is performed as follows:
- The site of the test is usually the volar or dorsal surface of the forearm about 4" below the elbow. Other skin sites may be used, but the volar surface of the forearm is preferred. The use of a skin area free of lesions and away from any veins is recommended.7
- The skin at the injection site is cleansed with 70% alcohol and allowed to dry.
- The test material is administered with a tuberculin syringe (0.5 or 1.0 mL) fitted with a short (1/4 to 1/2") 27 gauge needle.
- A separate, sterile, single-use disposable syringe and needle should be used for each individual patient.
- The diaphragm of the vial-stopper should be wiped with 70% alcohol.
- The needle is inserted through the stopper diaphragm of the inverted vial. Exactly 0.1 mL is filled into the syringe with care being taken to exclude air bubbles and to maintain the lumen of the needle filled.
- The point of the needle is inserted into the most superficial layers of the skin with the needle bevel pointed upward. As the Tuberculin solution is injected, a pale bleb 6 to 10 mm in size (1/3") will rise over the point of the needle. This is quickly absorbed and no dressing is required.
- There may be a drop of blood when the needle is withdrawn. This is normal. Use a gauze pad and gently dab to remove the blood. Do not press down as this may squeeze out the tuberculin thereby disrupting the test.
In the event the injection is delivered subcutaneously (i.e., no bleb will form), or if a significant part of the dose leaks from the injection site, the test should be repeated immediately at another site at least 5 cm (2") removed from the initial injection site.
Interpretation of Tuberculin Reaction
Readings of Mantoux reactions should be made by a trained health professional during the period from 48 to 72 hours after the injection. Induration only should be considered in interpreting the test. The diameter of induration should be measured transversely to the long axis of the forearm and recorded in millimeters. Erythema has no diagnostic value and should be disregarded. The presence and size of necrosis and edema if present should be recorded although not used in the interpretation of the test. In the absence of induration, an area of erythema greater than 10 mm in diameter may indicate the injection was made too deeply and retesting is indicated. Find the margins of the induration by drawing the index or middle finger lightly across the reaction. The tip of a ballpoint pen pushed at a 45° angle toward the site of injection will also stop at the edges of induration.
The diameter of induration should be measured (preferable with a caliper) transversely to the long axis of the forearm and recorded in millimeters.
Erythema has no diagnostic value and should be disregarded. The absence of induration should be recorded as "0 mm" not "negative".
Reactions should be interpreted as follows (Please refer to most recent guidelines):
Based on current guidelines,3,7,14, 19 interpretation of reactions is as follows:
Positive reactions
Reaction ≥ 5 mm of Induration Reaction ≥ 10 mm of Induration Reaction ≥ 15 mm of Induration Human immunodeficiency virus (HIV)-positive persons
Recent immigrants (i.e., within the last 5 yr) from high prevalence countries
Persons with no risk factors for TB
Recent contacts of tuberculosis (TB) case patients
Injection drug users
Fibrotic changes on chest radiograph consistent with prior TB
Residents and employees*of the following high-risk congregate settings: prisons and jails, nursing homes and other long-term facilities for the elderly, hospitals and other health care facilities, residential facilities for patients with acquired immunodeficiency syndrome (AIDS), and homeless shelters
Patients with organ transplants and other immunosuppressed patients (receiving the equivalent of ≥ 15 mg/d of prednisone for 1 mo or more)†
Mycobacteriology laboratory personnel
Persons with the following clinical conditions that place them at high risk: silicosis, diabetes mellitus, chronic renal failure, some hematological disorders (e.g., leukemias and lymphomas), other specific malignancies (e.g., carcinoma of the head or neck and lung), weight loss of ≥ 10% of ideal body weight, gastrectomy, and jejunoileal bypass
Children younger than 4 yr of age or infants, children, and adolescents exposed to adults at high-risk
Skin test conversions
- For persons with negative skin test reactions who undergo repeat skin testing (e.g., health care workers), an increase in reaction size ≥ 10 mm within a period of 2 years should be considered a skin test conversion indicative of recent infection with M. tuberculosis. 19
- In some individuals who have been infected with nontuberculous mycobacteria or have undergone BCG vaccination, the skin test may show some degree of induration. For these individuals, a conversion to "positive" is defined as an increase in induration by 10 mm on subsequent tests. 7
Healthcare facilities and other high-risk settings
- For health care workers and employees in other high-risk settings with no other risk factors for TB, a cut-off of 15 mm of induration (rather than 10 mm) on the tuberculin skin test should be used to define a positive baseline test at the time of initial employment.
- An increase of ≥10 mm in reaction size is generally accepted as a positive test result on subsequent testing unless the worker is a contact of a TB case or has HIV infection or is otherwise immunocompromised, in which case a result of ≥5 mm is considered positive. 21
Negative Reaction
A negative reaction is an induration of less than 15 mm in persons with no risk factors for TB. This indicates a lack of hypersensitivity to tuberculoprotein and tuberculous infection is highly unlikely.7
It should be noted that reactivity to tuberculin may be depressed or suppressed for as long as 5–6 weeks by viral infections, live virus vaccines (i.e., measles, smallpox, polio, rubella and mumps), or after discontinuation of therapy with corticosteroids or immunosuppressive agents. Malnutrition may also have a similar effect (see WARNINGS). When of diagnostic importance, a negative test should be accepted as proof that hypersensitivity is absent only after normal reactivity to non-specific irritants has been demonstrated. A primary injection of tuberculin may possibly have a boosting effect on subsequent tuberculin reactions. A pediatric patient who is known to have been exposed to a person with tuberculosis must not be adjudged free of infection until that patient has a negative tuberculin reaction at least ten weeks after contact with tuberculous person has ceased.17 Annual testing is generally recommended for pediatric patients in high risk populations, such as persons from countries with a high prevalence of tuberculosis and low-income groups.18
A positive tuberculin reaction does not necessarily signify the presence of active disease. Further diagnostic procedures (e.g., chest radiograph, sputum smear and/or culture examination) should be carried out before a diagnosis of tuberculosis is made. A small percentage of responders may not have been infected with M. tuberculosis but by some other mycobacterium. The negative tuberculin skin test should never be used to exclude the possibility of active tuberculosis among persons for whom the diagnosis is being considered (symptoms compatible with tuberculosis).
Booster Effect and Two-Step Testing
Infection of an individual with tubercle bacilli or other mycobacteria or BCG vaccination results in a delayed hypersensitivity response to tuberculin which is demonstrated by the skin test. The delayed hypersensitivity response may gradually wane over a period of years. If a person receives a tuberculin test at this time, a significant reaction may not be detected. However, the stimulus of the test may boost or increase the size of the reaction to a second test, sometimes causing an apparent conversion or development of sensitivity. This booster effect can be seen on a second test done one week after the initial stimulating test and can persist for a year, and perhaps longer. When routine periodic tuberculin testing of adults is done, initially two-stage testing should be considered to minimize the likelihood of interpreting a boosted reaction as a conversion.7,15,16
In this testing method, persons who have a negative initial skin test undergo a second tuberculin skin test 1-3 weeks after the first. Both tests should be read and recorded at 48 to 72 hours. Those individuals with a positive reaction on the second test should be considered to be previously infected, and those with a negative reaction on the second test should be considered uninfected. In these uninfected persons, a positive result on any future skin test should be interpreted as a skin test conversion. 7
-
HOW SUPPLIED
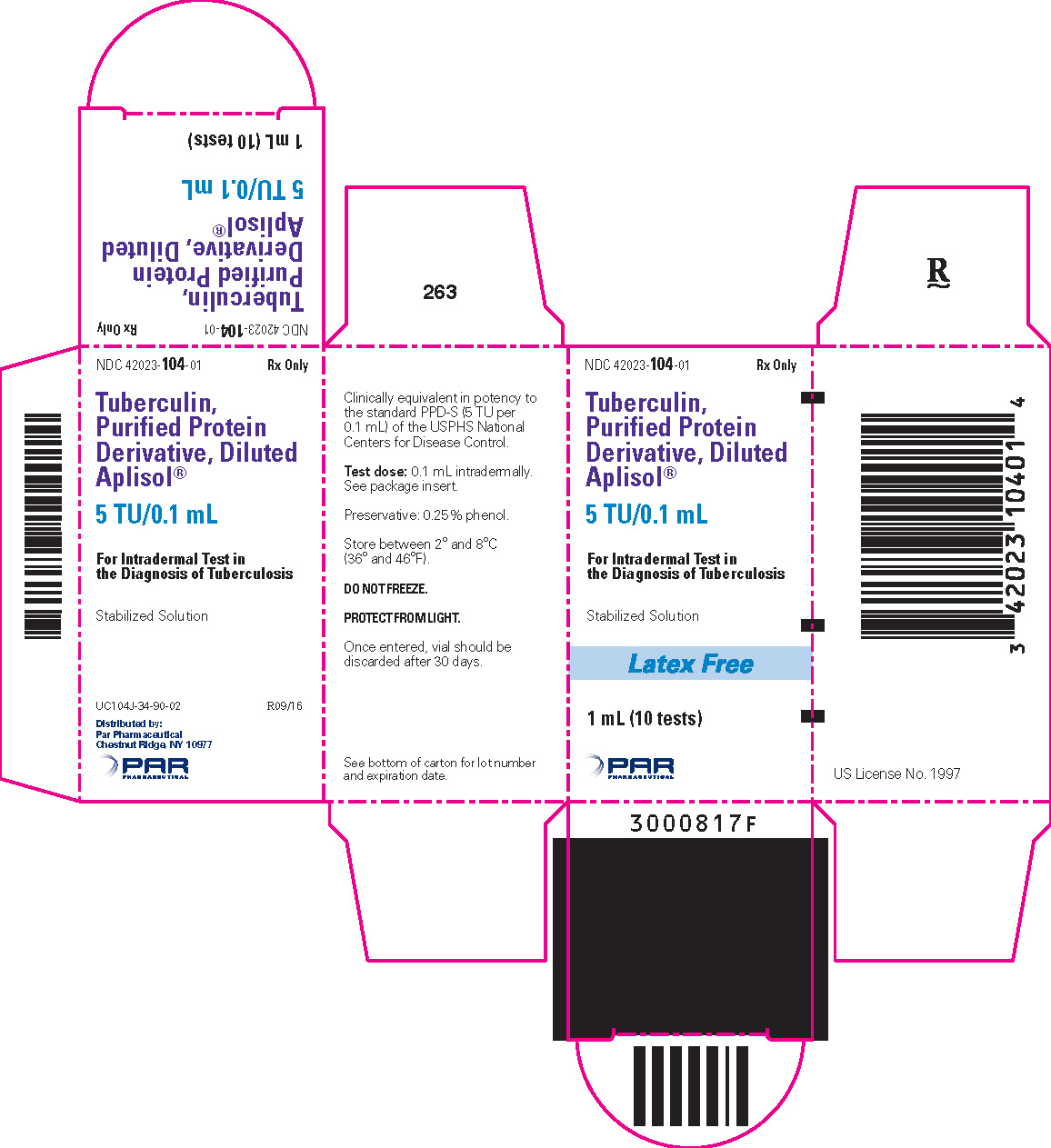
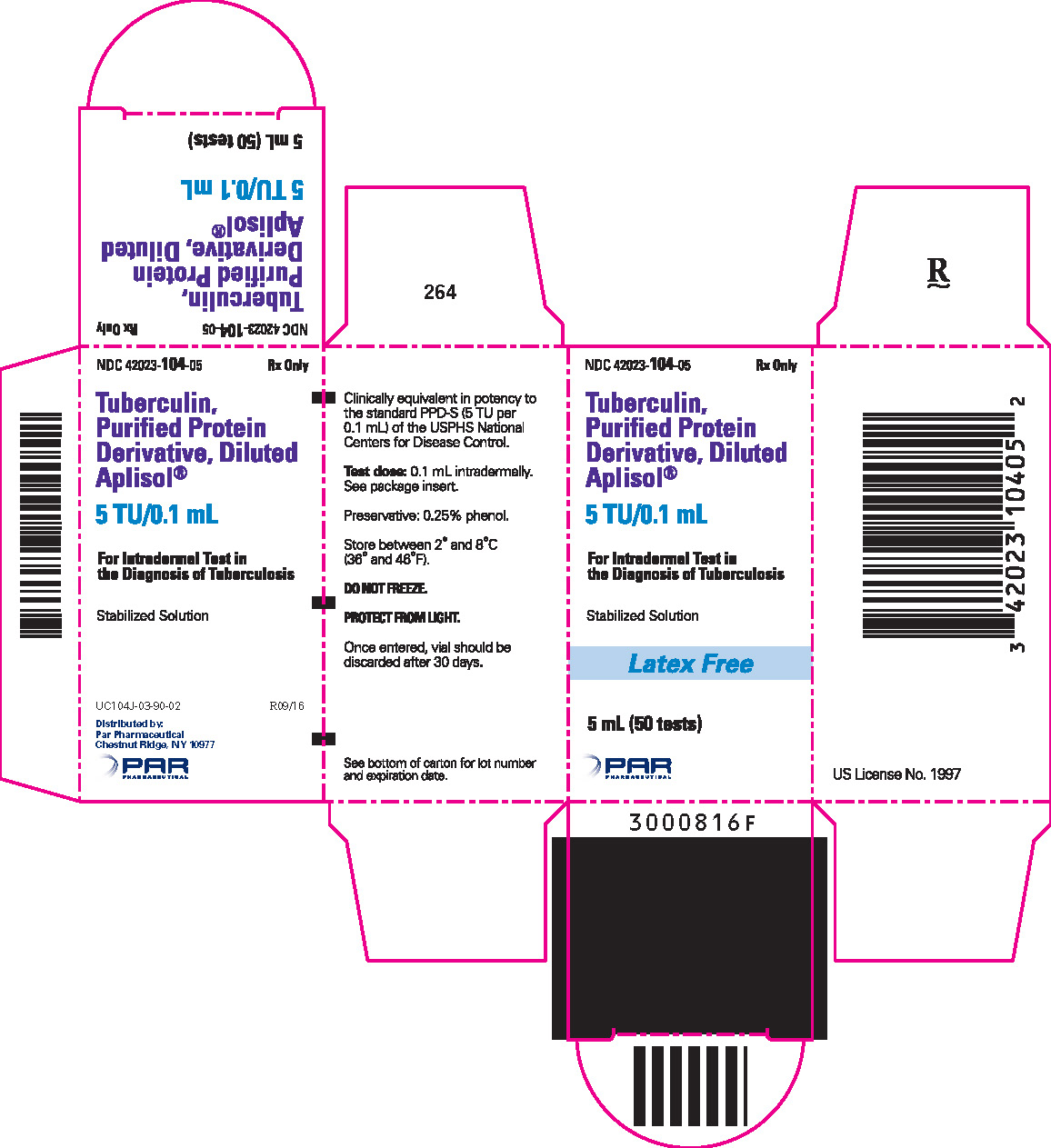
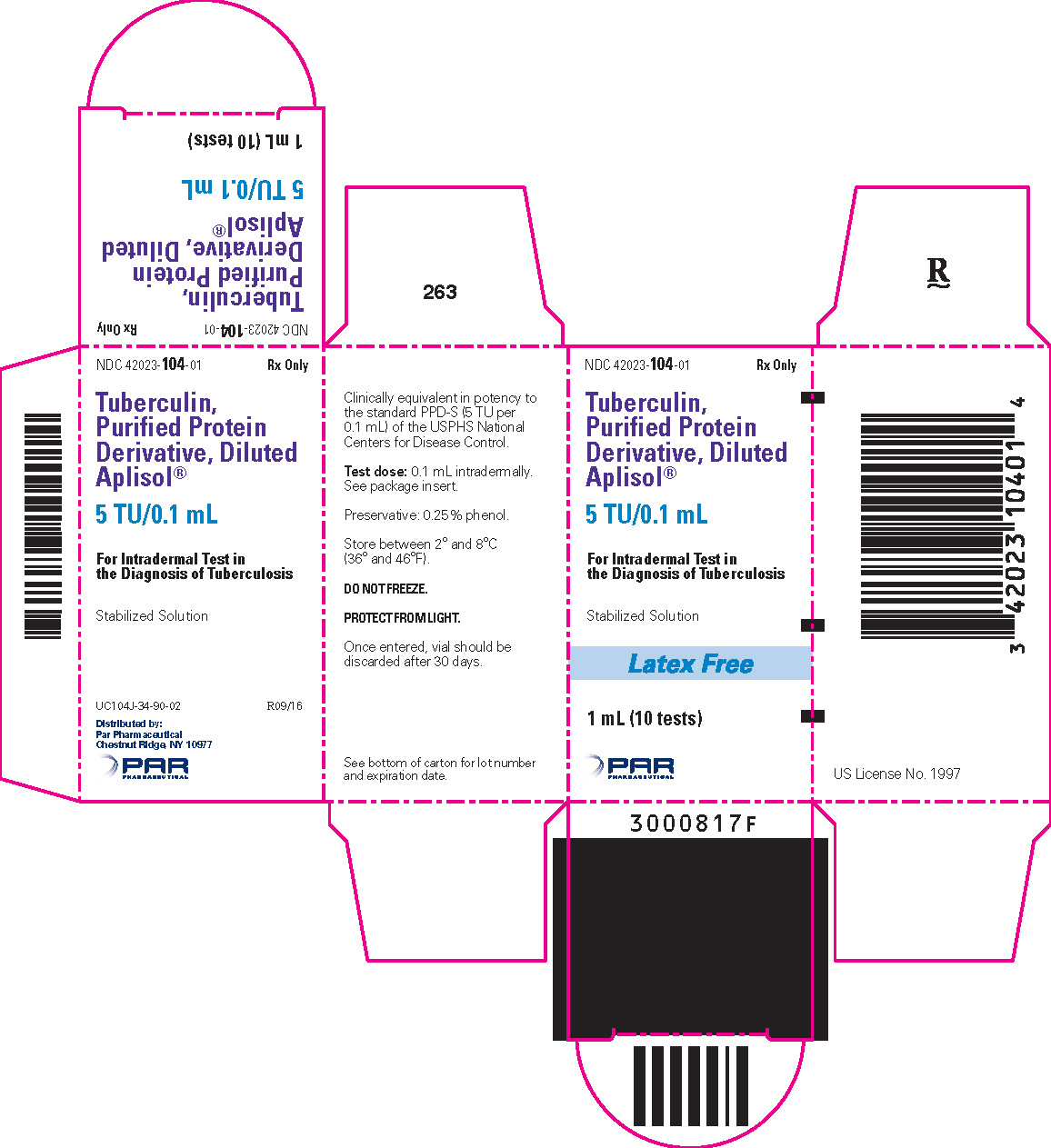
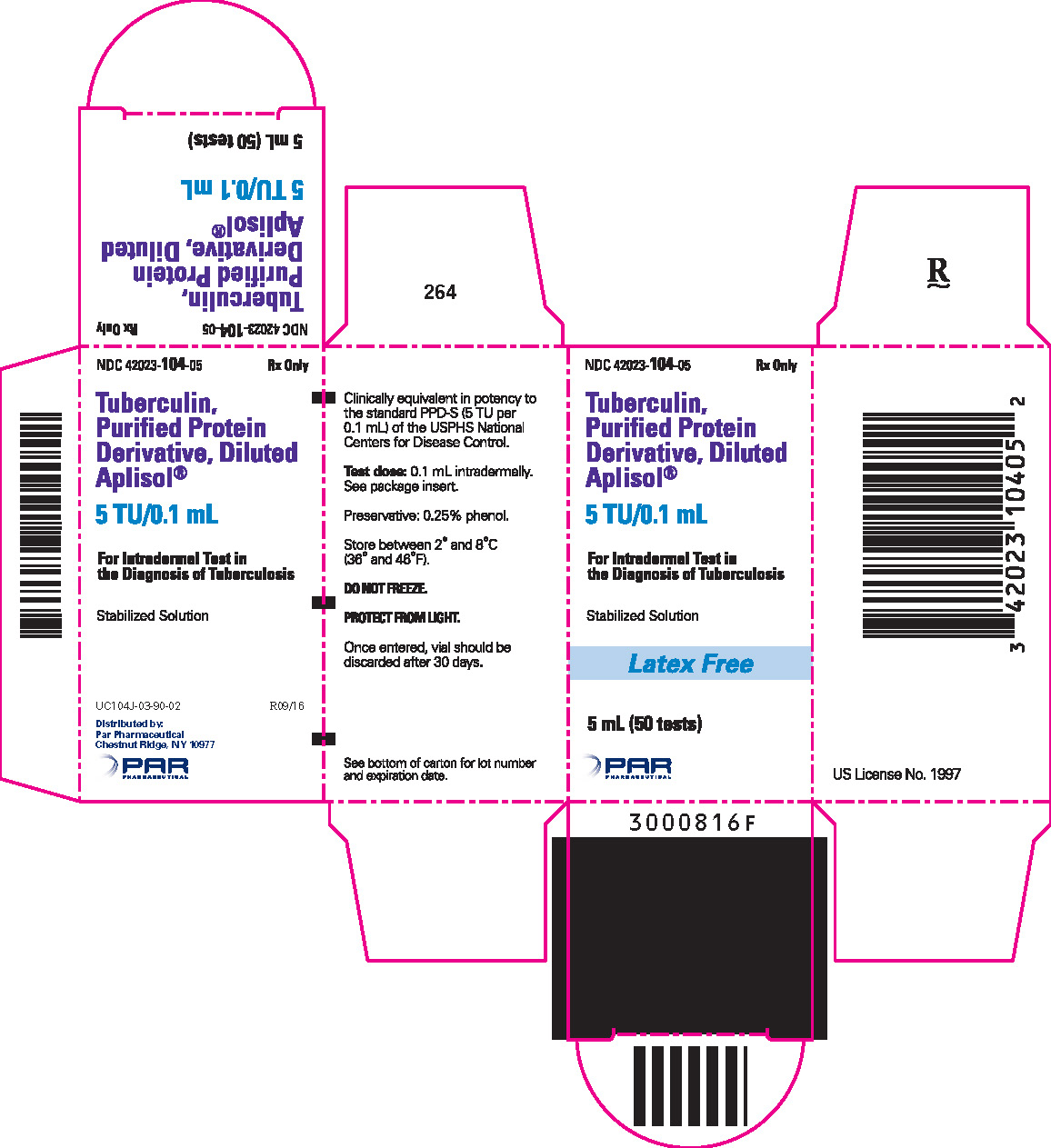
Tuberculin PPD-Aplisol bioequivalent to 5 US units (TU) PPD-S per test dose (0.1 mL) is available in the following presentations:
NDC 42023-104-01 (Bio. 1525)
1 mL (10 tests) – multiple dose vial
NDC 42023-104-05 (Bio.1607)
5 mL (50 tests) – multiple dose vial
This product is ready for use without further dilution.
-
REFERENCES
- Seibert, F.B. The isolation and properties of the purified protein derivative of tuberculin: Am Rev Tuberc 1934; 30:713.
- Seibert, F.B., and Glenn, J.T. Tuberculin purified protein derivative. Preparation and analyses of a large quantity for standard. Am Rev Tuberc 1941; 44:9-25.
- Centers for Disease Control and Prevention (CDC). Essential components of a tuberculosis prevention and control program; and Screening for tuberculosis and tuberculosis infection in high-risk populations: recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR 1995;44(RR-11):1-34.
- Centers for Disease Control and Prevention (CDC). Prevention and control of tuberculosis in correctional facilities: recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR 1996;45(RR-8):1-27.
- Huebner RE, Shein MF, Bass JB. The Tuberculin Skin Test. Clin Infect Dis 1993;17:968–75.
- AHFS Drug Information, 1997, 36:84 pp 1962–1968.
- American Thoracic Society: Diagnostic standards and classification of tuberculosis in Adults and Children. Am J Respir Crit Care Med 2000 Apr; 161:1376-95.
- Am Rev Respir Dis 1985;886.
- Brickman HF et.Al. The Timing of Tuberculin Tests in Relation to Immunization with Live Viral Vaccines. Pediatrics 1975;55:392.
- Nakayama K, Monma M, Fukushima T, Ohrui T, Sasaki H. Tuberculin responses and risk of pneumonia in immobile elderly patients. Thorax 2000 Oct;55(10):867-9.
- Fukushima T, Nakayama K, Monma M, Sekizawa K, Sasaki H. Depression of T helper-1 and the tuberculin responses in older bed-bound patients. J Am Geriatri Soc 1999 Feb;47(2):259-260.
- American Academy of Pediatrics. Tuberculosis. In: Pickering LK, CJ Baker, Long SS, McMillan JA, eds. Red Book: 2006 Report of the Committee on Infectious Diseases, 27th ed. Elk Grove Village, IL: American Academy of Pediatrics 2006: 678-698.
- Landi S, Held HR. Stability of a dilute solution of tuberculin purified derivative at extreme temperatures. J Biol Stand 1981; 9:195.
- Centers For Disease Control and Prevention (CDC). Diagnosis of TB Infection and TB Disease, March 21,1996, Doc# 2250102.
- Sewell, E.M., O'Hare, D., and Kendig, E.L., Jr. The Tuberculin Test. Pediatrics Vol. 54, No. 5, Nov.1974.
- Centers for Disease Control and Prevention(CDC). Prevention and control of tuberculosis in facilities providing long-term care to the elderly. Recommendations of the Advisory Committee of Elimination of Tuberculosis (ACET): MMWR 1990, 39(RR-10): 7–20.
- Centers for Disease Control and Prevention(CDC). The use of preventative therapy for tuberculosis infection in the United States, Recommendations of the Advisory Committee of Elimination of Tuberculosis (ACET), MMWR 1990 39(RR-8):9–12.
- Centers for Disease Control and Prevention(CDC). Screening for tuberculosis and tuberculosis infection in high risk populations. Recommendations of the Advisory Committee of Elimination of Tuberculosis (ACET), MMWR 1990, 39(RR-8): 1-7.
- Centers for Disease Control and Prevention (CDC). Targeted Tuberculin Testing and Treatment of Latent Tuberculosis Infection. MMWR, 2000. 49(RR-6): 1-51.
- Pediatrics: Screening for Tuberculosis in Infants and Children, 1994. 93: 131-134.
- Centers for Disease Control and Prevention (CDC). Controlling Tuberculosis in the United States. MMWR 2005. 54 (RR-12).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1 mL Vial Carton
- PRINCIPAL DISPLAY PANEL - 5 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
APLISOL
tuberculin purified protein derivative injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42023-104 Route of Administration INTRADERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TUBERCULIN PURIFIED PROTEIN DERIVATIVE (UNII: I7L8FKN87J) (TUBERCULIN PURIFIED PROTEIN DERIVATIVE - UNII:I7L8FKN87J) TUBERCULIN PURIFIED PROTEIN DERIVATIVE 5 [iU] in 0.1 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) PHENOL (UNII: 339NCG44TV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42023-104-01 1 in 1 CARTON 04/25/2008 1 1 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC:42023-104-05 1 in 1 CARTON 04/25/2008 2 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103782 04/25/2008 Labeler - Par Pharmaceutical, Inc. (092733690)