Label: PAPAVERINE HYDROCHLORIDE injection
- NDC Code(s): 14789-121-05, 14789-121-07
- Packager: Nexus Pharamaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Papaverine Hydrochloride, USP, is the hydrochloride of an alkaloid obtained from opium or prepared synthetically. It belongs to the benzylisoquinoline group of alkaloids. It does not contain a phenanthrene group as do morphine and codeine.
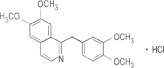
Papaverine Hydrochloride, USP, is 6,7-dimethoxy-1- veratrylisoquinoline hydrochloride and contains, on the dried basis, not less than 98.5% of C20H21NO4•HCl. The molecular weight is 375.85. The structural formula is as shown.
Papaverine Hydrochloride occurs as white crystals or white crystalline powder. One gram dissolves in about 30 mL of water and in 120 mL of alcohol. It is soluble in chloroform and practically insoluble in ether.
Papaverine Hydrochloride Injection, USP, is a clear, colorless to pale-yellow solution.
Papaverine Hydrochloride, for parenteral administration, is a smooth-muscle relaxant that is available in vials containing 30 mg/mL. Each vial also contains edetate disodium 0.005%.
pH may be adjusted with sodium citrate and/or citric acid.
-
CLINICAL PHARMACOLOGY
The most characteristic effect of papaverine is relaxation of the tonus of all smooth muscle, especially when it has been spasmodically contracted. Papaverine Hydrochloride apparently acts directly on the muscle itself. This relaxation is noted in the vascular system and bronchial musculature and in the gastrointestinal, biliary and urinary tracts.
The main actions of papaverine are exerted on cardiac and smooth muscle. Papaverine relaxes various smooth muscles, especially those of larger arteries; this relaxation may be prominent if spasm exists. The antispasmodic effect is a direct one and unrelated to muscle innervation, and the muscle still responds to drugs and other stimuli causing contraction. Papaverine has minimal actions on the central nervous system, although very large doses tend to produce some sedation and sleepiness in some patients. In certain circumstances, mild respiratory stimulation can be observed, but this is therapeutically inconsequential. Papaverine stimulates respiration by acting on carotid and aortic body chemoreceptors.
Papaverine relaxes the smooth musculature of the larger blood vessels, including the coronary, cerebral, peripheral, and pulmonary arteries. This action is particularly evident when such vessels are in spasm, induced reflexly or by drugs, and it provides the basis for the clinical use of papaverine in peripheral or pulmonary arterial embolism.
Experimentally in dogs, the alkaloid has been shown to cause fairly marked and long-lasting coronary vasodilatation and an increase in coronary blood flow. However, it also appears to have a direct inotropic effect and, when increased mechanical activity coincides with decreased systemic pressure, increases in coronary blood flow may not be sufficient to prevent brief periods of hypoxic myocardial depression.
Papaverine is effective by all routes of administration. A considerable fraction of the drug localizes in fat deposits and in the liver, with the remainder being distributed throughout the body. It is metabolized in the liver. About 90% of the drug is bound to plasma protein. Although estimates of its biologic half-life vary widely, reasonably constant plasma levels can be maintained with oral administration at 6 hour intervals. The drug is excreted in the urine in an inactive form.
-
INDICATIONS AND USAGE
Papaverine is recommended in various conditions accompanied by spasm of smooth muscle, such as vascular spasm associated with acute myocardial infarction (coronary occlusion), angina pectoris, peripheral and pulmonary embolism, peripheral vascular disease in which there is a vasospastic element, or certain cerebral angiospastic states; and visceral spasm, as in ureteral, biliary, or gastrointestinal colic.
-
CONTRAINDICATIONS
Intravenous injection of papaverine is contraindicated in the presence of complete atrioventricular heart block. When conduction is depressed, the drug may produce transient ectopic rhythms of ventricular origin, either premature beats or paroxysmal tachycardia.
Papaverine Hydrochloride is not indicated for the treatment of impotence by intracorporeal injection. The intracorporeal injection of papaverine hydrochloride has been reported to have resulted in persistent priapism requiring medical and surgical intervention.
-
PRECAUTIONS
General - Papaverine Hydrochloride Injection, USP, should not be added to Lactated Ringer's Injection, because precipitation would result.
Papaverine Hydrochloride should be used with caution in patients with glaucoma. The medication should be discontinued if hepatic hypersensitivity with gastrointestinal symptoms, jaundice, or eosinophilia becomes evident or if liver function test values become altered.
Usage In Pregnancy - Pregnancy Category C - No teratogenic effects were observed in rats when papaverine hydrochloride was administered subcutaneously as a single agent. It is not known whether papaverine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Papaverine Hydrochloride should be given to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
The following side effects have been reported: general discomfort, nausea, abdominal discomfort, anorexia, constipation or diarrhea, skin rash, malaise, vertigo, headache, intensive flushing of the face, perspiration, increase in the depth of respiration, increase in heart rate, a slight rise in blood pressure, and excessive sedation.
Hepatitis, probably related to an immune mechanism, has been reported infrequently. Rarely, this has progressed to cirrhosis.
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
Signs and Symptoms - The symptoms of toxicity from papaverine hydrochloride often result from vasomotor instability and include nausea, vomiting, weakness, central nervous system depression, nystagmus, diplopia, diaphoresis, flushing, dizziness, and sinus tachycardia. In large overdoses, papaverine is a potent inhibitor of cellular respiration and a weak calcium antagonist. Following an oral overdose of 15 g, metabolic acidosis with hyperventilation, hyperglycemia, and hypokalemia have been reported. No information on toxic serum concentrations is available.
Following intravenous overdosing in animals, seizures, tachyarrhythmias, and ventricular fibrillation have been reported. The oral median lethal dose in rats is 360 mg/kg.
Treatment - To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physician's Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
Protect the patient's airway and support ventilation and perfusion. Meticulously monitor vital signs, blood gases, blood chemistry values, and other variables.
If convulsions occur, consider diazepam, phenytoin, or phenobarbital. If the seizures are refractory, general anesthesia with thiopental or halothane and paralysis with a neuromuscular blocking agent may be necessary.
For hypotension, consider intravenous fluids, elevation of the legs, and an inotropic vasopressor, such as dopamine or norepinephrine (levarterenol). Theoretically, calcium gluconate may be helpful in treating some of the toxic cardiovascular effects of papaverine; monitor the ECG and plasma calcium concentrations.
Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for an overdose of papaverine hydrochloride.
-
DOSAGE AND ADMINISTRATION
Papaverine Hydrochloride may be administered intravenously or intramuscularly. The intravenous route is recommended when an immediate effect is desired, but the drug must be injected slowly over the course of 1 or 2 minutes to avoid uncomfortable or alarming side effects.
Parenteral administration of papaverine hydrochloride in doses of 1 to 4 mL is repeated every 3 hours as indicated. In the treatment of cardiac extrasystoles, 2 doses may be given 10 minutes apart.
-
HOW SUPPLIED:
Papaverine Hydrochloride Injection, USP, 30 mg/mL
NDC: 14789-121-07 2 mL Single-dose Vial
NDC: 14789-121-05 10 pack carton of 2 mL Single-dose Vials
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
PROTECT FROM LIGHT. RETAIN IN CARTON UNTIL TIME OF USE.
Container closer was not made with natural rubber latex.
Manufactured in the USA by:
Nexus Pharmaceuticals, Inc. Lincolnshire, IL 60069
PAPPI01R02 Revised 03/2023
NEXUS
PHARMACEUTICALS - Principal Display Panel – 2 mL Carton Label
- Principal Display Panel – 2 mL Vial Label
-
INGREDIENTS AND APPEARANCE
PAPAVERINE HYDROCHLORIDE
papaverine hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:14789-121 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Papaverine Hydrochloride (UNII: 23473EC6BQ) (Papaverine - UNII:DAA13NKG2Q) Papaverine Hydrochloride 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength Edetate Disodium (UNII: 7FLD91C86K) 0.05 mg in 1 mL Sodium Citrate (UNII: 1Q73Q2JULR) Citric Acid Monohydrate (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14789-121-05 10 in 1 CARTON 06/28/2021 1 NDC:14789-121-07 2 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/28/2021 Labeler - Nexus Pharamaceuticals Inc. (620714787) Establishment Name Address ID/FEI Business Operations Nexus Pharmaceuticals Inc 620714787 ANALYSIS(14789-121)