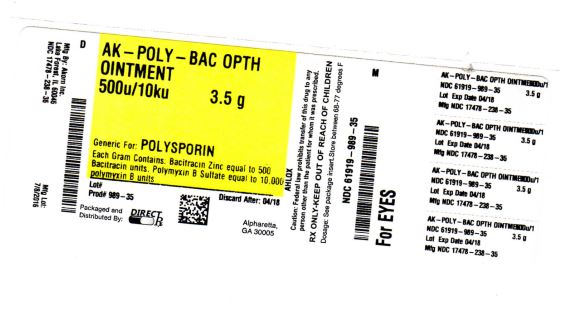
Label: AK-POLY-BAC ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 61919-989-35 - Packager: DIRECT RX
- This is a repackaged label.
- Source NDC Code(s): 17478-238
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 17, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION SECTION
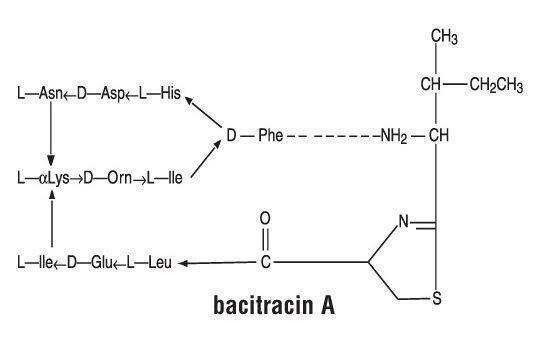
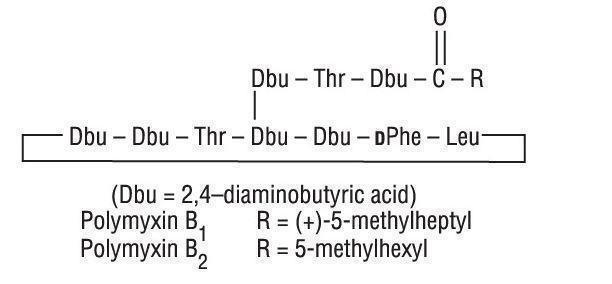
Polymyxin B Sulfate is the sulfate salt of polymyxin B1 and B2 which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units per mg, calculated on an anhydrous basis. The structural formulae are:
Each gram contains: Bacitracin zinc equal to 500 bacitracin units and polymyxin B sulfate equal to 10,000 polymyxin B units, white petrolatum and mineral oil.
- CLINICAL PHARMACOLOGY SECTION
- INDICATIONS & USAGE SECTION
- CONTRAINDICATIONS SECTION
- WARNINGS SECTION
- PRECAUTIONS SECTION
- DOSAGE & ADMINISTRATION SECTION
-
HOW SUPPLIED SECTION
Bacitracin Zinc and Polymyxin B Sulfate ophthalmic ointment USP, sterile, each gram contains bacitracin zinc equal to 500 bacitracin units and polymyxin B sulfate equal to 10,000 polymyxin B units, in a tube of 3.5 g (1/8 oz) with ophthalmic tip.
NDC 17478-238-35
STORAGE:
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Akorn
Manufactured By: Akorn, Inc.
Lake Forest, IL 60045
AKP00N
Rev. 11/08
Principal Display Panel Text for Container Label:
NDC 17478-238-35 Akorn
AK-POLY-BAC™
brand of Bacitracin Zinc and Polymyxin B Sulfate
Ophthalmic Ointment USP
For Ophthalmic Use Only. Sterile
Rx only Net Wt. 3.5 g (1/8oz.)
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AK-POLY-BAC
ak-poly-bac ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61919-989(NDC:17478-238) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [USP'U] in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61919-989-35 3.5 g in 1 CARTON; Type 0: Not a Combination Product 01/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA064028 01/01/2014 Labeler - DIRECT RX (079254320) Establishment Name Address ID/FEI Business Operations DIRECT RX 079254320 relabel(61919-989)