Label: MATA PIOJOS LICE- piperonyl butoxide, pyrethrum extract shampoo
- NDC Code(s): 48201-006-08, 48201-006-59
- Packager: GRANDALL DISTRIBUTING, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
near eyes
inside nose, mouth, or vagina
on lice in eyebrows or eyelashes. See a doctor if lice are present in these areas.
Ask a doctor before use if you are
• allergic to ragweed. May cause breathing difficulty or an asthmatic attack.
When using this product
keep eyes tightly closed and protect eyes with a washcloth or towel
if product gets into eyes, flush with water right away
scalp itching or redness may occur
-
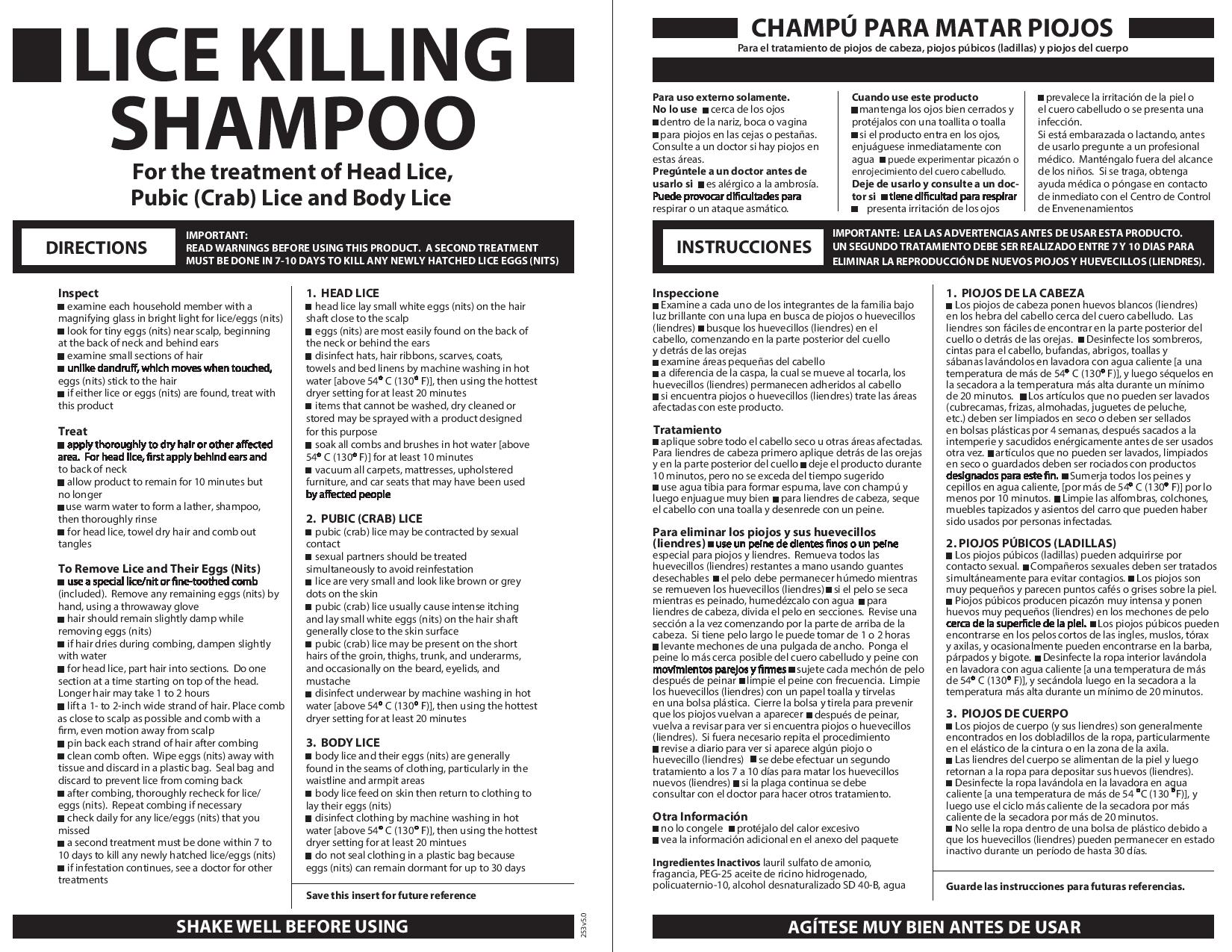
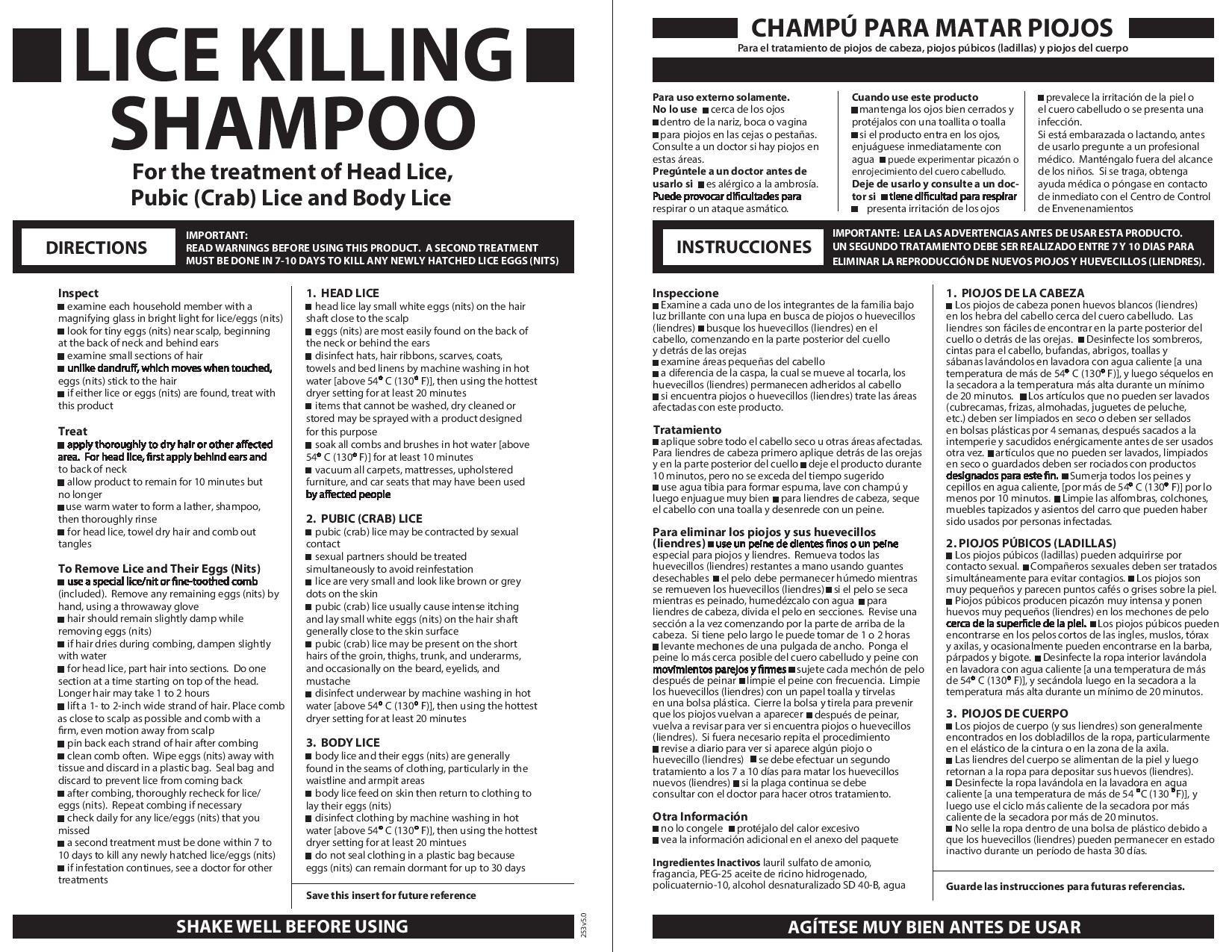
Directions
• Important: Read warnings before use. Adults and children 2 years and over: Inspect•check each household member with a magnifying glass in bright light for lice/nits (eggs)•look for tiny nits near scalp, beginning at back of neck and behind ears•examine small sections of hair at a time•unlike dandruff which moves when touched, nits stick to the hair•if either lice or nits are found, treat with this product. Treat•apply thoroughly to DRY HAIR or other affected area. For head lice, first apply behind ears and to back of neck.•allow product to remain for 10 minutes, but no longer•use warm water to form a lather, shampoo, then thoroughly rinse•for head lice, towel dry hair and comb out tangles. Remove lice and their eggs (nits)•use a fine-tooth or special lice/nit comb. Remove any remaining nits by hand (using a throw-away glove).hair should remain slightly damp while removing nitsif hair dries during combing, dampen slightly with waterfor head lice, part hair into sections. Do one section at a time starting on top of the head. Longer hair may take 1 to 2 hours.lift a 1-to 2-inch wide strand of hair. Place comb as close to scalp as possible and comb with a firm, even motion away from scalp.pin back each strand of hair after combing•clean comb often. Wipe nits away with tissue and discard in a plastic bag. Seal bag and discard to prevent lice from coming back.•after combing, thoroughly recheck for lice/nits. Repeat combing if necessary.•check daily for any lice/nits that you missed•a second treatment must be done in 7 to 10 days to kill any newly hatched lice•if infestation continues, see a doctor for other treatments•Children under 2 years: ask a doctor
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MATA PIOJOS LICE
piperonyl butoxide, pyrethrum extract shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:48201-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PIPERONYL BUTOXIDE (UNII: LWK91TU9AH) (PIPERONYL BUTOXIDE - UNII:LWK91TU9AH) PIPERONYL BUTOXIDE 4 g in 100 mL PYRETHRUM EXTRACT (UNII: ZUM06L90GV) (PYRETHRUM EXTRACT - UNII:ZUM06L90GV) PYRETHRUM EXTRACT 0.33 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:48201-006-59 1 in 1 CARTON 07/09/2015 1 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:48201-006-08 1 in 1 CARTON 06/01/2018 2 237 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M031 07/09/2015 Labeler - GRANDALL DISTRIBUTING, LLC (044428324)