Label: CLINDAMYCIN HYDROCHLORIDE capsule
- NDC Code(s): 60760-827-30
- Packager: ST. MARY'S MEDICAL PARK PHARMACY
- This is a repackaged label.
- Source NDC Code(s): 68462-144
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of clindamycin hydrochloride and other antibacterial drugs, clindamycin hydrochloride should be used only to ...
-
BOXED WARNING
(What is this?)
WARNING
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including clindamycin hydrochloride and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon, leading to overgrowth of C. difficile.
Because clindamycin hydrochloride therapy has been associated with severe colitis which may end fatally, it should be reserved for serious infections where less toxic antimicrobial agents are inappropriate, as described in the INDICATIONS AND USAGEsection. It should not be used in patients with nonbacterial infections such as most upper respiratory tract infections.
C. difficileproduces toxins A and B, which contribute to the development of CDAD. Hypertoxin producing strains of C. difficilecause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficilemay need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Close -
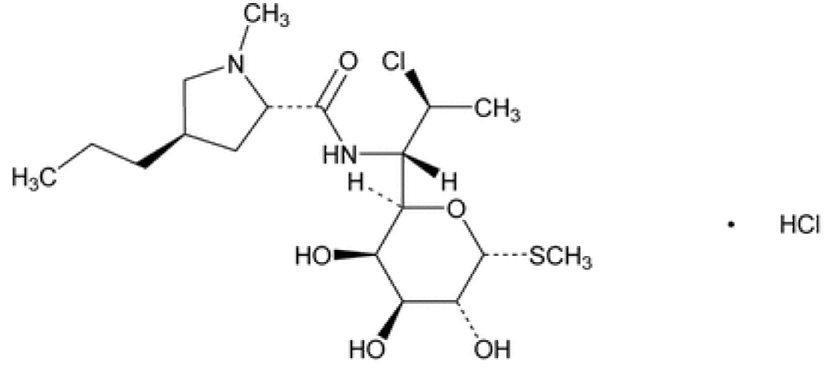
DESCRIPTIONClindamycin hydrochloride, USP is the hydrated hydrochloride salt of clindamycin. Clindamycin is a semisynthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the ...
-
CLINICAL PHARMACOLOGYHuman Pharmacology - Absorption - Pharmacokinetic studies with a 150 mg oral dose of clindamycin hydrochloride in 24 normal adult volunteers showed that clindamycin was rapidly absorbed after oral ...
-
INDICATIONS AND USAGEClindamycin is indicated in the treatment of serious infections caused by susceptible anaerobic bacteria. Clindamycin is also indicated in the treatment of serious infections due to susceptible ...
-
CONTRAINDICATIONSClindamycin hydrochloride capsules are contraindicated in individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin.
-
WARNINGSSee - BOXED WARNING - Clostridioides difficile-Associated Diarrhea - Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents ...
-
PRECAUTIONSGeneral - Review of experience to date suggests that a subgroup of older patients with associated severe illness may tolerate diarrhea less well. When clindamycin is indicated in these patients ...
-
ADVERSE REACTIONSThe following reactions have been reported with the use of clindamycin. Infections and Infestations:Clostridioides difficilecolitis - Gastrointestinal:Abdominal pain, pseudomembranous colitis ...
-
OVERDOSAGESignificant mortality was observed in mice at an intravenous dose of 855 mg/kg and in rats at an oral or subcutaneous dose of approximately 2618 mg/kg. In the mice, convulsions and depression were ...
-
DOSAGE AND ADMINISTRATIONIf significant diarrhea occurs during therapy, this antibiotic should be discontinued (see - BOXED WARNING). Adults: Serious infections– 150 to 300 mg every 6 hours. More severe ...
-
HOW SUPPLIEDClindamycin Hydrochloride Capsules, USP are available in the following strengths, colors and sizes: Clindamycin Hydrochloride Capsules, USP 300 mg are size ‘0’ hard gelatin capsules with a light ...
-
REFERENCES1. Smith RB, Phillips JP: Evaluation of CLEOCIN HCl and CLEOCIN Phosphate in an Aged Population. Upjohn TR 8147-82-9122-021, December 1982. Trademarks are the property of their respective ...
-
PRINCIPAL DISPLAY PANEL
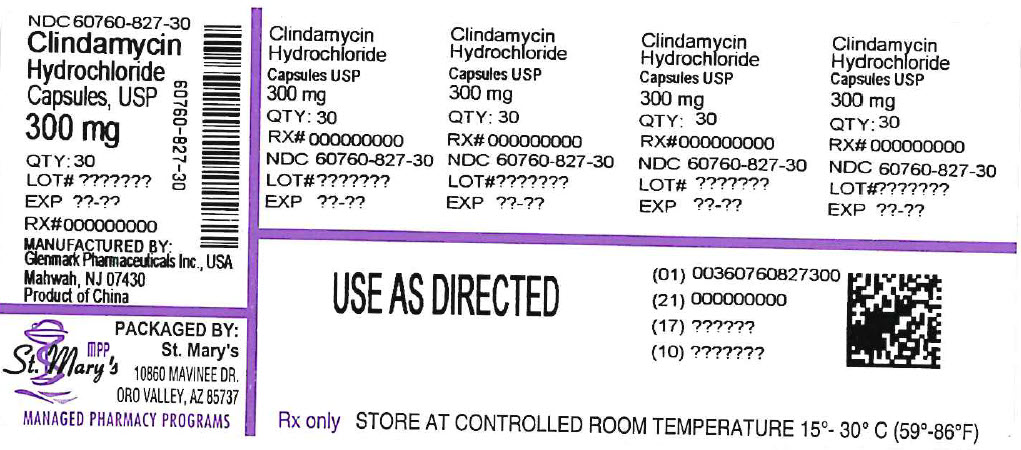
-
INGREDIENTS AND APPEARANCEProduct Information