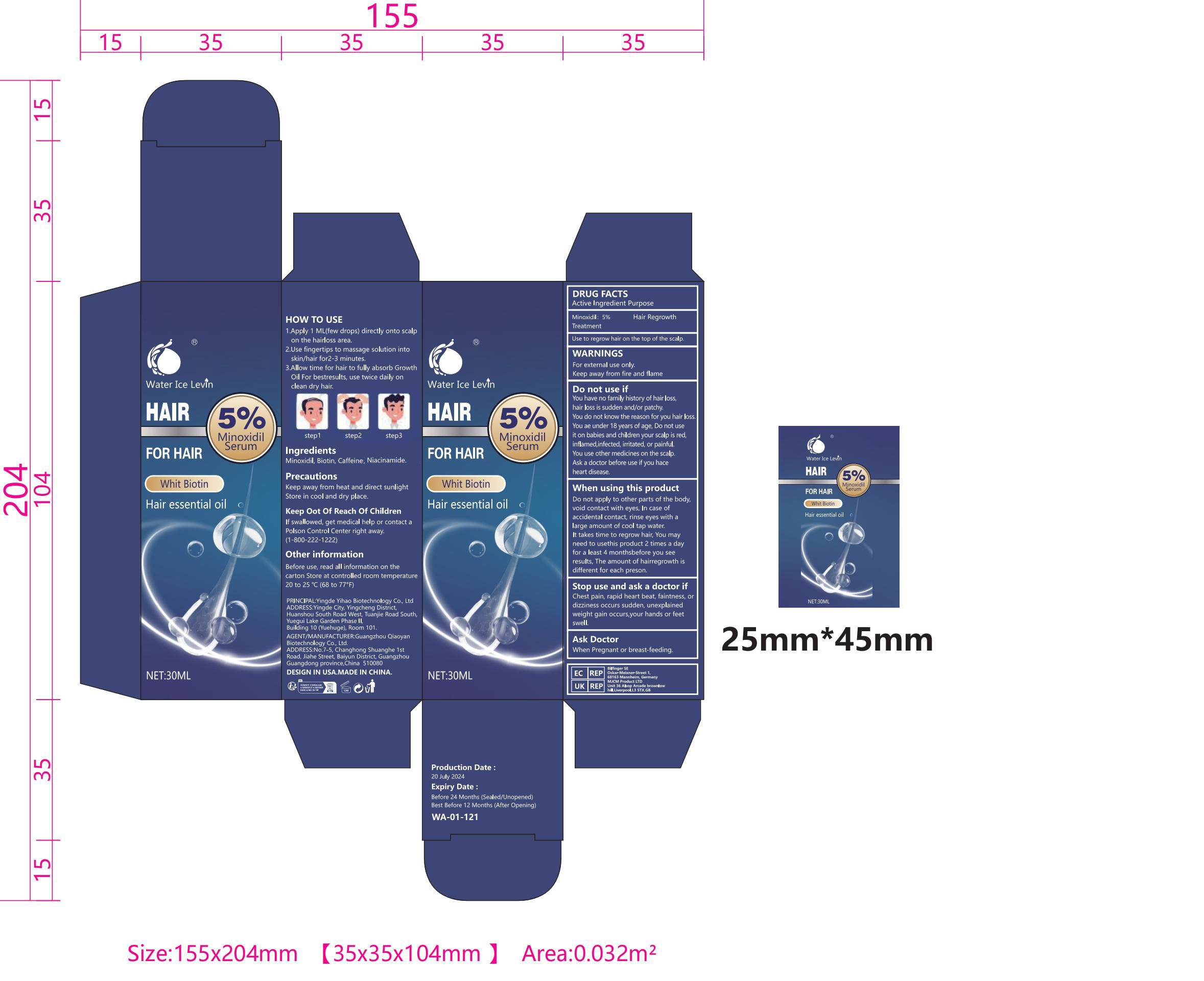
Label: WATER ICE LEVIN WHIT BIOTIN HAIR ESSENTIAL OIL liquid
- NDC Code(s): 84550-001-01
- Packager: Guangzhou Qiaoyan Biotechnology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTSActive Ingredient Purpose
- Purpose
- Purpose
- Uses
- WARNINGS
- Dosage and administration
-
Do not use if
Do not apply to other parts of the bodyvoid contact with eyes, In case ofaccidental contact, rinse eyes with alarge amount of cool tap water.It takes time to regrow hair, You mayneed to usethis product 2 times a dayfor a least 4 monthsbefore you seeresults, The amount of hairregrowth isdifferent for each preson.
-
When using this product
Do not apply to other parts of the body,void contact with eyes, In case ofaccidental contact, rinse eyes with alarge amount of cool tap water.It takes time to regrow hair, You mayneed to usethis product 2 times a dayfor a least 4 monthsbefore you seeresults, The amount of hairregrowth isdifferent for each preson.
- Stop use and ask a doctor if
- Keep Oot Of Reach of children
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WATER ICE LEVIN WHIT BIOTIN HAIR ESSENTIAL OIL
water ice levin whit biotin hair essential oil liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84550-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 g Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) CAFFEINE (UNII: 3G6A5W338E) BIOTIN (UNII: 6SO6U10H04) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84550-001-01 1 g in 1 BOX; Type 0: Not a Combination Product 07/19/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 07/19/2024 Labeler - Guangzhou Qiaoyan Biotechnology Co., Ltd (402888513) Establishment Name Address ID/FEI Business Operations Guangzhou Qiaoyan Biotechnology Co., Ltd 402888513 manufacture(84550-001)