Label: IMBUE PAIN RELIEF- menthol - methyl salicylate patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 75979-1146-4 - Packager: Imbue Body LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 1, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
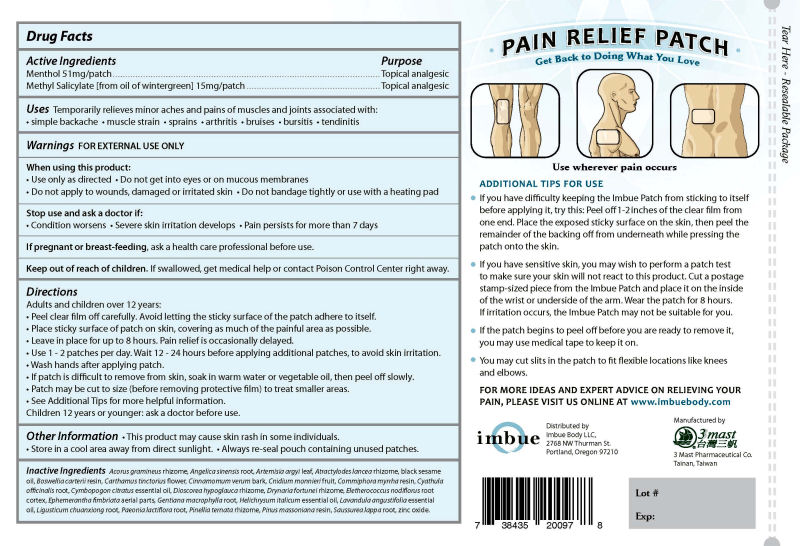
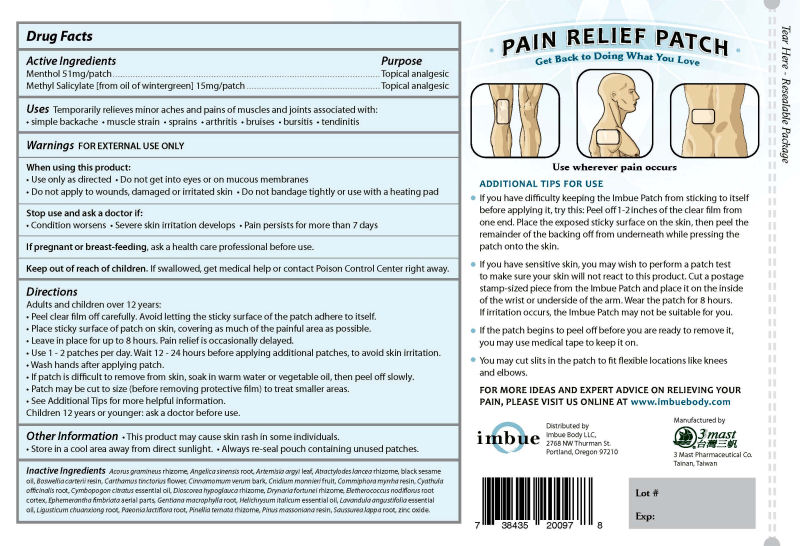
Directions
Adults and children over 12 years:
• Peel clear film off carefully. Avoid letting the sticky surface of the patch adhere to itself.
• Place sticky surface of patch on skin, covering as much of the painful area as possible.
• Leave in place for up to 8 hours. Pain relief is occasionally delayed.
• Use 1 - 2 patches per day. Wait 12 - 24 hours before applying additional patches, to avoid skin irritation.
• Wash hands after applying patch.
• If patch is difficult to remove from skin, soak in warm water or vegetable oil, then peel off slowly.
• Patch may be cut to size (before removing protective film) to treat smaller areas.
• See Additional Tips for more helpful information.
Children 12 years or younger: ask a doctor before use.
- SPL UNCLASSIFIED SECTION
-
INACTIVE INGREDIENT
Inactive Ingredients: Acorus gramineus rhizome, Angelica sinensis root, Artemisia argyi leaf, Atractylodes lancea rhizome, black sesame oil, Boswellia carterii resin, Carthamus tinctorius flower, Cinnamomum verum bark, Cnidium monnieri fruit, Commiphora myrrha resin, Cyathula officinalis root, Cymbopogon citratus essential oil, Dioscorea hypoglauca rhizome, Drynaria fortunei rhizome, Eletherococcus nodiflorus root cortex, Ephemerantha fimbriata aerial parts, Gentiana macrophylla root, Helichrysum italicum essential oil, Lavandula angustifolia essential oil, Ligusticum chuanxiong root, Paeonia lactiflora root, Pinellia ternata rhizome, Pinus massoniana resin, Saussurea lappa root, zinc oxide.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IMBUE PAIN RELIEF
menthol - methyl salicylate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75979-1146 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Menthol (UNII: L7T10EIP3A) (Menthol - UNII:L7T10EIP3A) Menthol 51 mg Methyl salicylate (UNII: LAV5U5022Y) (salicylic acid - UNII:O414PZ4LPZ) Methyl salicylate 15 mg Inactive Ingredients Ingredient Name Strength Acorus gramineus root (UNII: Z60N6Q6E19) Angelica sinensis root (UNII: B66F4574UG) Artemisia argyi leaf (UNII: 2JYC99Q0WZ) Atractylodes lancea root (UNII: CAZ6282J2O) Sesame oil (UNII: QX10HYY4QV) Frankincense (UNII: R9XLF1R1WM) Safflower (UNII: 4VBL71TY4Y) Cinnamon (UNII: 5S29HWU6QB) Cnidium monnieri fruit (UNII: V1IA3S3CUS) Myrrh (UNII: JC71GJ1F3L) Cyathula officinalis root (UNII: BRM37UP34O) West Indian lemongrass oil (UNII: 5BIA40E9ED) HELICHRYSUM ITALICUM FLOWER OIL (UNII: O97ZV7726K) LAVENDER OIL (UNII: ZBP1YXW0H8) LIGUSTICUM SINENSE SUBSP. CHUANXIONG ROOT (UNII: RR83T99U97) PAEONIA LACTIFLORA ROOT (UNII: 3Z3866YW6P) SAUSSUREA COSTUS ROOT (UNII: RUP970CGR9) Zinc oxide (UNII: SOI2LOH54Z) Dioscorea collettii var. hypoglauca root (UNII: N57RVD5L6U) Drynaria fortunei root (UNII: 731W842X8Q) Flickingeria fimbriata stem (UNII: D6E4A8IAEP) Gentiana macrophylla root (UNII: 6559FC0U1B) PINELLIA TERNATA ROOT (UNII: G9AET085M5) Pinus massoniana resin (UNII: 64S07U83T7) Rosin (UNII: 88S87KL877) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75979-1146-4 4 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 06/01/2011 Labeler - Imbue Body LLC (965843878) Establishment Name Address ID/FEI Business Operations Taiwan Three Mast Pharmaceutical Company 658305156 manufacture