Label: METFORMIN HYDROCHLORIDE EXTENDED RELEASE- metformin hydrochloride tablet, extended release
-
NDC Code(s):
49483-623-01,
49483-623-09,
49483-623-10,
49483-623-50, view more49483-624-01, 49483-624-50
- Packager: TIME CAP LABORATORIES, INC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 6, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
- HOW SUPPLIED
-
DOSAGE & ADMINISTRATION
There is no fixed dosage regimen for the management of hyperglycemia in patients with type 2 diabetes with metformin hydrochloride extended-release tablets or any other pharmacologic agent. Dosage of metformin hydrochloride extended-release tablets must be individualized on the basis of both effectiveness and tolerance, while not exceeding the maximum recommended daily doses. The maximum recommended daily dose of metformin hydrochloride extended-release tablets in adults is 2000 mg.
Metformin hydrochloride extended-release tablets should generally be given once daily with the evening meal. Metformin hydrochloride extended-release tablets should be started at a low dose, with gradual dose escalation, both to reduce gastrointestinal side effects and to permit identification of the minimum dose required for adequate glycemic control of the patient.
During treatment initiation and dose titration (see Recommended Dosing Schedule), fasting plasma glucose should be used to determine the therapeutic response to metformin hydrochloride extended-release tablets and identify the minimum effective dose for the patient. Thereafter, glycosylated hemoglobin should be measured at intervals of approximately three months. The therapeutic goal should be to decrease both fasting plasma glucose and glycosylated hemoglobin levels to normal or near normal by using the lowest effective dose of metformin hydrochloride extended-release tablets, either when used as monotherapy or in combination with sulfonylurea or insulin.
Monitoring of blood glucose and glycosylated hemoglobin will also permit detection of primary failure, i.e., inadequate lowering of blood glucose at the maximum recommended dose of medication, and secondary failure, i.e., loss of an adequate blood glucose lowering response after an initial period of effectiveness.
Short-term administration of metformin hydrochloride extended-release tablets may be sufficient during periods of transient loss of control in patients usually well-controlled on diet alone.
Metformin hydrochloride extended-release tablets must be swallowed whole and never crushed or chewed. Occasionally, the inactive ingredients of metformin hydrochloride extended-release tablets will be eliminated in the feces as a soft, hydrated mass. (See Patient information printed below.)
Recommended Dosing Schedule
Adults - In general, clinically significant responses are not seen at doses below 1500 mg per day. However, a lower recommended starting dose and gradually increased dosage is advised to minimize gastrointestinal symptoms.
The usual starting dose of metformin hydrochloride extended-release tablet is 500 mg once daily with the evening meal. Dosage increases should be made in increments of 500 mg weekly, up to a maximum of 2000 mg once daily with the evening meal. If glycemic control is not achieved on metformin hydrochloride extended-release tablets 2000 mg once daily, a trial of metformin hydrochloride extended-release tablets 1000 mg twice daily should be considered.
In a randomized trial, patients currently treated with metformin hydrochloride tablets were switched to metformin hydrochloride extended-release tablets. Results of this trial suggest that patients receiving metformin hydrochloride tablets treatment may be safely switched to metformin hydrochloride extended-release tablets once daily at the same total daily dose, up to 2000 mg once daily. Following a switch from metformin hydrochloride tablets to metformin hydrochloride extended-release tablets, glycemic control should be closely monitored and dosage adjustments made accordingly
Pediatrics – Safety and effectiveness of metformin hydrochloride extended-release tablets in pediatric patients have not been established.
Transfer From Other Antidiabetic Therapy
When transferring patients from standard oral hypoglycemic agents other than chlorpropamide to metformin hydrochloride extended-release tablets, no transition period generally is necessary. When transferring patients from chlorpropamide, care should be exercised during the first two weeks because of the prolonged retention of chlorpropamide in the body, leading to overlapping drug effects and possible hypoglycemia.
Concomitant Metformin Hydrochloride Extended-Release Tablets and Oral Sulfonylurea
Therapy in Adult Patients
If patients have not responded to four weeks of the maximum dose of metformin hydrochloride extended-release tablets monotherapy, consideration should be given to gradual addition of an oral sulfonylurea while continuing metformin hydrochloride extended-release tablets at the maximum dose, even if prior primary or secondary failure to a sulfonylurea has occurred. Clinical and pharmacokinetic drug-drug interaction data are currently available only for metformin plus glyburide (glibenclamide).
With concomitant metformin hydrochloride extended-release tablets and sulfonylurea therapy, the desired control of blood glucose may be obtained by adjusting the dose of each drug. However, attempts should be made to identify the minimum effective dose of each drug to achieve this goal. With concomitant metformin hydrochloride extended-release tablets and sulfonylurea therapy, the risk of hypoglycemia associated with sulfonylurea therapy continues and may be increased. Appropriate precautions should be taken. (See Package Insert of the respective sulfonylurea.)
If patients have not satisfactorily responded to one to three months of concomitant therapy with the maximum dose of metformin hydrochloride extended-release tablets and the maximum dose of an oral sulfonylurea, consider therapeutic alternatives including switching to insulin with or without metformin hydrochloride extended-release tablets.
Concomitant Metformin Hydrochloride Extended-Release Tablets and Insulin Therapy in Adult PatientsThe current insulin dose should be continued upon initiation of metformin hydrochloride extended-release tablets therapy. Metformin hydrochloride extended-release tablets therapy should be initiated at 500 mg once daily in patients on insulin therapy. For patients not responding adequately, the dose of metformin hydrochloride extended-release tablets should be increased by 500 mg after approximately 1 week and by 500 mg every week thereafter until adequate glycemic control is achieved. The maximum recommended daily dose is 2000 mg for metformin hydrochloride extended-release tablets. It is recommended that the insulin dose be decreased by 10% to 25% when fasting plasma glucose concentrations decrease to less than 120 mg/dL in patients receiving concomitant insulin and metformin hydrochloride extended-release tablets. Further adjustment should be individualized based on glucose-lowering response.
Specific Patient Populations
Metformin hydrochloride extended-release tablets are not recommended for use in pregnancy. Metformin hydrochloride extended-release tablet is not recommended in pediatric patients (below the age of 17 years).
The initial and maintenance dosing of metformin hydrochloride extended-release tablets should be conservative in patients with advanced age, due to the potential for decreased renal function in this population. Any dosage adjustment should be based on a careful assessment of renal function. Generally, elderly, debilitated, and malnourished patients should not be titrated to the maximum dose of metformin hydrochloride extended-release tablets.
Monitoring of renal function is necessary to aid in prevention of lactic acidosis, particularly in the elderly. -
WARNINGS
WARNING: LACTIC ACIDOSIS
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metforminassociated lactic acidosis was characterized by elevated blood lactate levels (>5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL (see PRECAUTIONS).
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g. carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided (see DOSAGE AND ADMINISTRATION, CONTRAINDICATIONS, and PRECAUTIONS).
If metformin-associated lactic acidosis is suspected, immediately discontinue metformin and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended (see PRECAUTIONS).
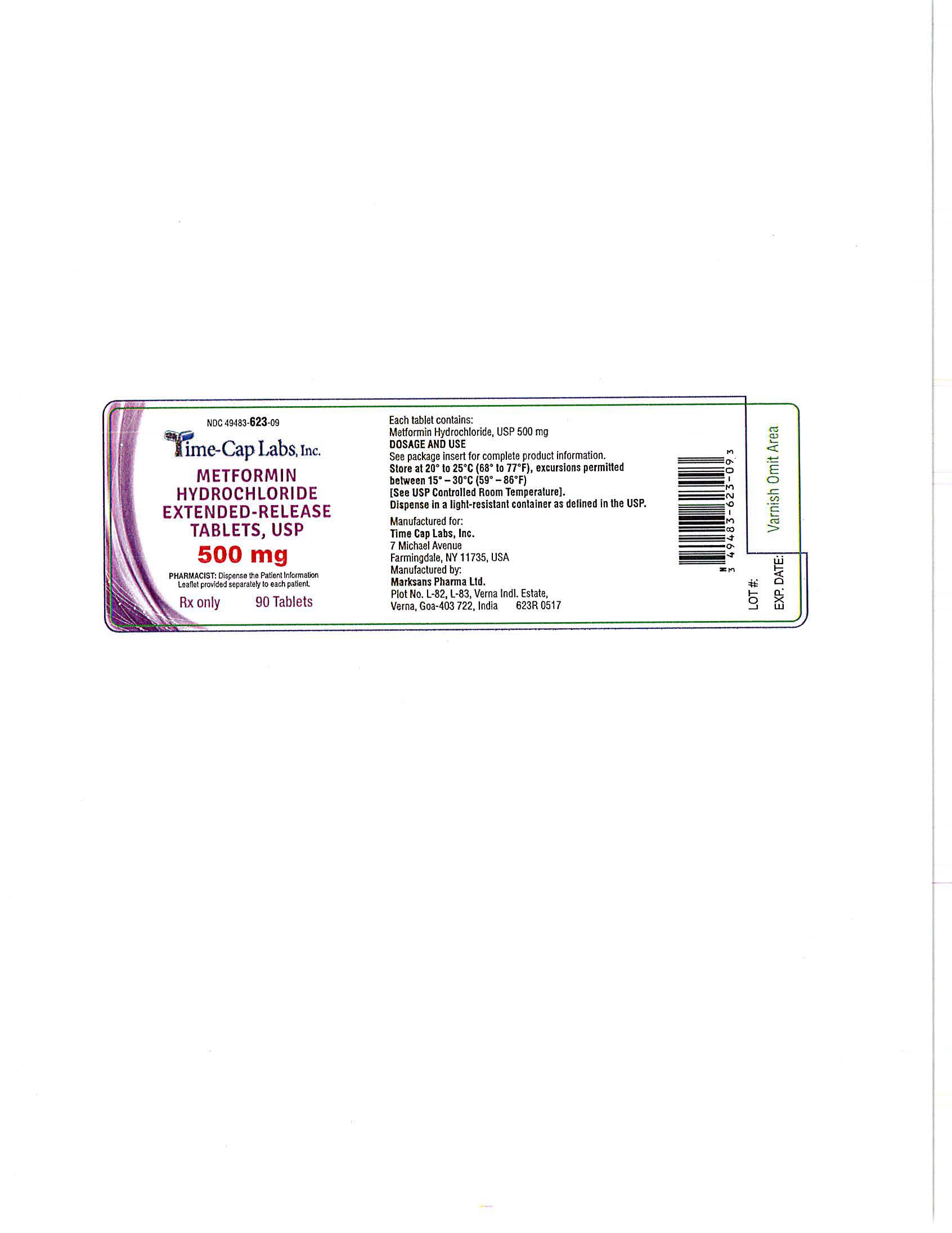
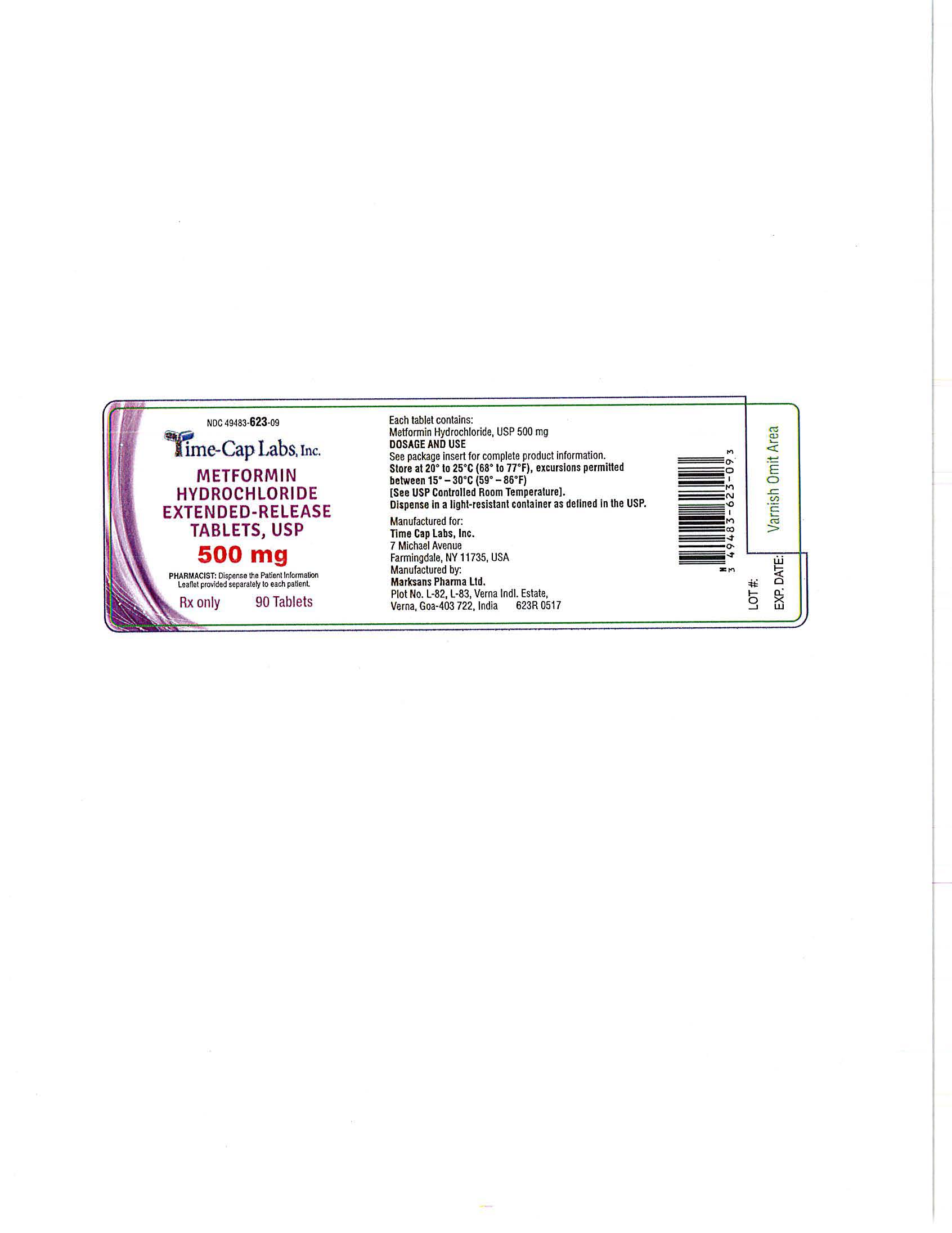
- METFORMIN ER TABLETS 500 MG - 90 LABEL
- METFORMIN ER TABLETS 500 MG - 100 LABEL
- METFORMIN ER TABLETS 500 MG -500 LABEL
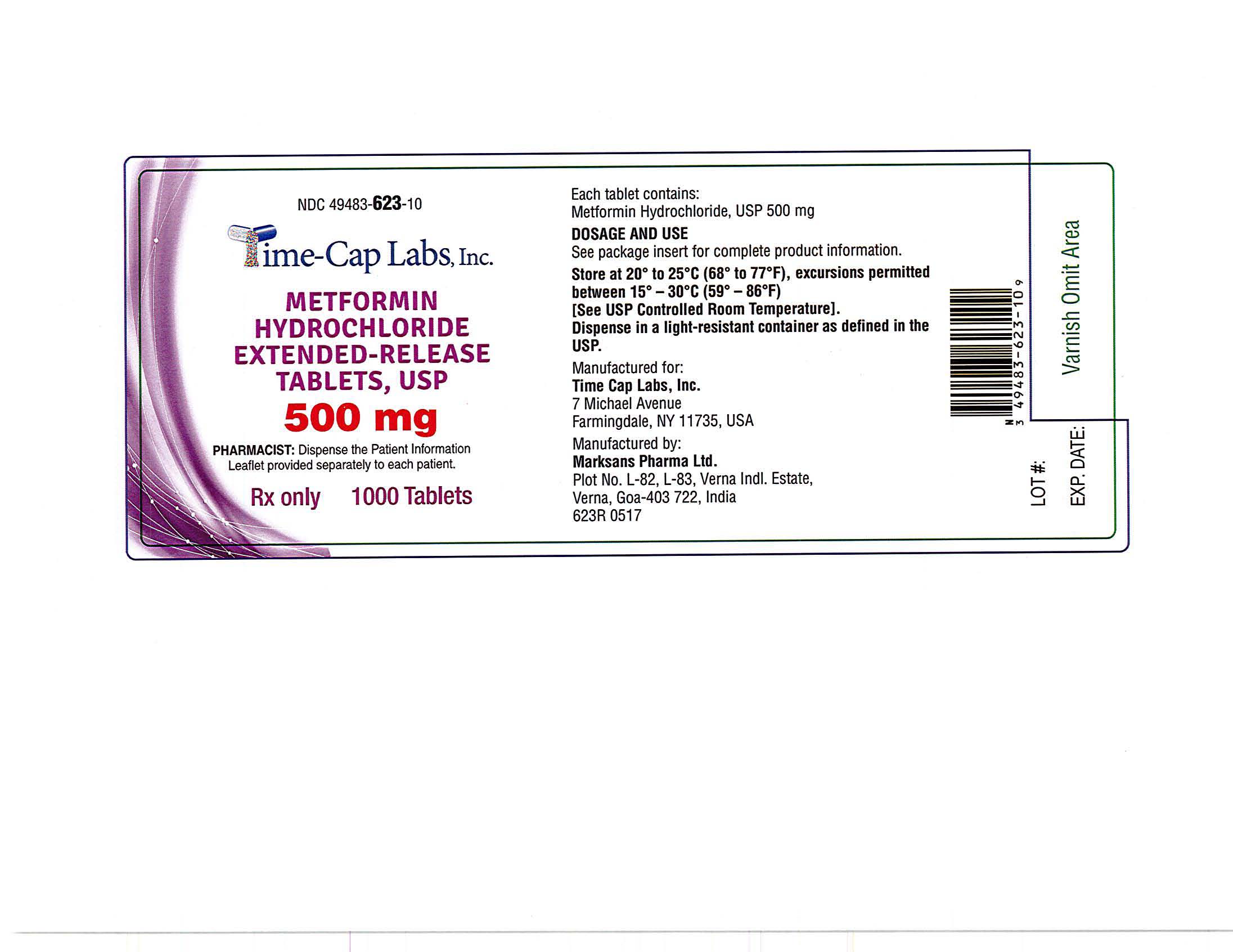
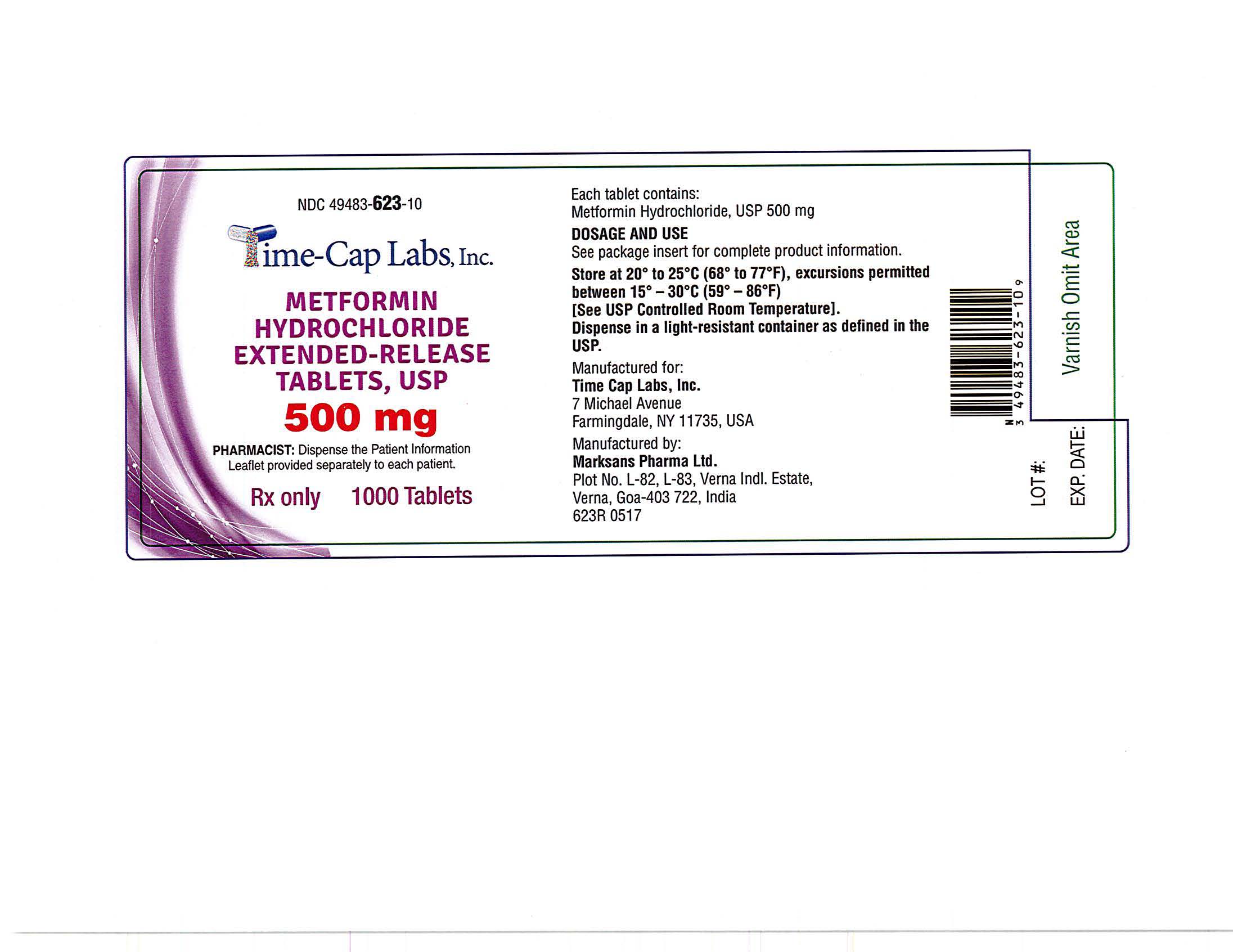
- METFORMIN ER TABLETS 500 MG -1000 LABEL
- METFORMIN ER TABLETS 750 MG 100 LABEL
- METFORMIN ER TABLETS 750 MG 500 LABEL
-
INGREDIENTS AND APPEARANCE
METFORMIN HYDROCHLORIDE EXTENDED RELEASE
metformin hydrochloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49483-623 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 500 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 101 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49483-623-09 90 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/05/2017 2 NDC:49483-623-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/28/2016 3 NDC:49483-623-50 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/28/2016 4 NDC:49483-623-10 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/05/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090295 05/12/2016 METFORMIN HYDROCHLORIDE EXTENDED RELEASE
metformin hydrochloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49483-624 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 750 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape CAPSULE Size 19mm Flavor Imprint Code 102 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49483-624-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/12/2016 2 NDC:49483-624-50 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/12/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090295 05/12/2016 Labeler - TIME CAP LABORATORIES, INC (037052099) Registrant - TIME CAP LABORATORIES, INC (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 925822975 manufacture(49483-623, 49483-624)